Abstract
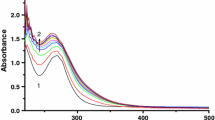
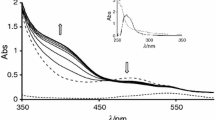
The interaction of di-μ-hydroxobis(bipyridyl)dipalladium(II) with the selected bio-relevant ligands: thioglycolic acid (HL1), glutathione(HL2) and DL-penicillamine(HL3) has been studied spectrophotometrically in aqueous medium as a function of complex and ligand concentrations, pH, and temperature at constant ionic strength. Two consecutive reaction steps are observed: the first step is dependent on the incoming ligand concentration and the second step which is independent, is assigned to ring closure. The activation parameters, conductivity data and IR data obtained also support the proposed mechanism.
Similar content being viewed by others
References
Shoukry, M.M., Shehata, M.R., Abdel-Razik, A., Abdel-Karim, A.T.I.: Equilibrium studies of mixed ligand complexes involving (1,2-diaminopropane)-palladium(II) and some bioligands. Mon. für Chem. 130, 409–423 (1999)
El-Medani, S.M., Shohayeb, M., Shoukry, M.M.: Equilibrium studies of complex formation involving palladium(II)-(1,3-diaminopropane) and cyclobutanedicarboxylic acid, DNA units, amino acids or peptides. Transit. Met. Chem. 23, 287–293 (1998)
Shoukry, M.M., van Eldik, R.: Correlation between kinetic and thermodynamic complex-formation constants for the interaction of bis(amine)palladium(II) with inosine, inosine 5′-monophosphate and guanosine 5′-monophosphate. J. Chem. Soc., Dalton Trans., 2673–2678 (1996)
Shoukry, M.M., Hohmann, H., van Eldik, R.: Mechanistic information on the reaction of model palladium(II) complexes with purine nucleosides and 5′-nucleotides in reference to the antitumor activity of related platinum complexes. Inorg. Chim. Acta 198, 187–192 (1992)
Shehata, M.R.: Mixed ligand complexes of diaquo (2,2′-bipyridine)palladium(II) with cyclobutane-1,1-dicarboxylic acid and DNA constituents. Transit. Met. Chem. 26, 198–204 (2001)
Rau, T., van Eldik, R.: In: Sigel, H., Sigel, A. (eds.) Metal Ions in Biological Systems, vol. 31, pp. 339–378. Marcel Dekker, New York (1996)
Lippert, B. (ed.): Cisplatin-chemistry and Biochemistry of Leading Anticancer Drugs, Wiley VCH, Zürich (1999)
Reedijk, J.: Why does cisplatin reach guanine-N7 with competing S-donor ligands available in the cell? Chem. Rev. 99, 2499–2510 (1999)
Jamieson, E.R., Lippard, S.J.: Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 99, 2467–2498 (1999)
Karakoff, I.H.: In: Nicolini, M. (ed.) Platinum and Other Metal Coordination Compounds in Cancer Chemotherapy; Clinical Applications of Platinum Complexes, p. 351. Martinus Nijhoff, Boston (1988)
Wong, E., Giandornenico, C.M.: Current status of platinum-based antitumor drugs. Chem. Rev. 99, 2451–2466 (1999)
Gökce, M., Utku, S., Gür, S., Özkul, A., Gümus, F.: Synthesis in vitro cytotoxic and antiviral activity of cis-[Pt(R(–) and S(+)-2-α-hydroxybenzylbenzimidazole)2Cl2] complexes. Eur. J. Med. Chem. 40, 135–141 (2005)
McCormick, B.J., Jaynes(jun), E.N., Kaplan, R.I.: Dichloro(ethylenediamine)palladium(II) and (2,2′-bipyridine)dichloropalladium(II). Inorg. Synth. 13, 216–218 (1972)
Bennet, G.M., Masses, A.N., Stathan, F.S.: CCXVIII.-Stereoisomerism of disulphoxides and related substances. Part VI. Co-ordination compounds of some disulphides and diamines. J. Chem. Soc., 1668–1676 (1930)
Hay, R.W., Basak, A.K.: Hydrolysis of α-amino-acid esters in mixed-ligand complexes with 2,2′-bipyridylpalladium(II). J. Chem. Soc. Dalton Trans., 1819–1823 (1982)
Nakamoto, K.: Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th edn. Wiley-Interscience, New York (1986)
Maltese, M., Orville-Thomas, W.J.: The infrared spectra and structure of some complex hydroxosalts. J. Inorg. Nucl. Chem. 29, 2533–2544 (1967)
Williams, R.L., Pace, R.J.: Notes. J. Chem. Soc., 4131–4156 (1957)
Baran, V.: Hydroxyl ion as a ligand. Coord. Chem. Rev. 6, 65–93 (1971)
Marchal, S., Moreno, V., Aullon, G., Alvarez, S., Quiros, M., Font-Bardia, M., Solans, X.: Synthesis and study of trinuclear Pd(II) and Pt(II) complexes with 2-mercaptonicotinic acid: Crystal and molecular structure of [Pd3(mercaptonicotinic acid)3Cl3]. Polyhedron 18, 3675–3682 (1999)
Weyh, J.A., Hamm, R.E.: Aquation of the cis-bis(iminodiacetato)chromate(III) and trans(fac)-bis(methyliminodiacetato)chromate(III) ions in acidic aqueous medium. Inorg. Chem. 8, 2298–2302 (1969)
Stability Constants, Special publication, No. 17, p. 376. The Chemical Society, London (1964)
Rabenstein, D.L.: Nuclear magnetic resonance studies of the acid-base chemistry of amino acids and peptides. I. Microscopic ionization constants of glutathione and methylmercury-complexed glutathione. J. Am. Chem. Soc. 95, 2797–2803 (1973)
Smith, R.M., Martell, A.E.: Critical Stability Constants, 2nd edn., vol. 6, p. 21. Plenum Press, New York (1989)
Anderegg, G., Wanner, H.: Pyridine derivatives as complexing agents. XIII. The stability of the palladium(II) complexes with pyridine, 2,2′-bipyridyl, and 1,10-phenanthroline. Inorg. Chim. Acta 113, 101–108 (1986)
Wimmer, F.L., Wimmer, S.: On the stability of palladium(II) aquo complexes with 2,2′−bipyridyl and 1,10-phenanthroline. Inorg. Chim. Acta 149, 1–3 (1988)
Wimmer, S., Castan, P., Wimmer, F.L., Johnson, N.P.: Aqueous chemistry of Pt(II) and Pd(II) complexes of 2,2′-bipyridine and 1,10-phenanthroline: pH dependence. Inorg. Chim. Acta 142, 13–15 (1988)
Wimmer, S., Castan, P., Wimmer, F.L., Johnson, N.P.: Preparation and interconversion of dimeric di-μ-hydroxo and tri-μ-hydroxo complexes of platinum(II) and palladium(II) with 2,2′-bipyridine and 1,10-phenanthroline. J. Chem. Soc., Dalton Trans., 403–412 (1989)
EL-Sherif, A.A., Shoukry, M.M., van Eldik, R.: Complex-formation reactions and stability constants for mixed-ligand complexes of diaqua(2-picolylamine)palladium(II) with some bio-relevant ligands. J. Chem. Soc., Dalton Trans., 1425–1432 (2003)
Mallick, S., Bera, B.K., Mandal, A., Karmakar, P., Ghosh, A.K.: Kinetic and mechanistic studies on the interaction of DL-penicillamine with di-μ-hydroxobis(bipyridyl)dipalladium(II) ion in aqueous solution. J. Indian Chem. Soc. (2011, in press)
Zhu, L., Kostic, N.M.: Toward artificial metallopeptidases: mechanisms by which platinum(II) and palladium(II) complexes promote selective, fast hydrolysis of unactivated amide bonds in peptides. Inorg. Chem. 31, 3994–4001 (1992)
Zhu, L., Kostic, N.M.: Hydrolytic cleavage of peptides by palladium(II) complexes is enhanced as coordination of peptide nitrogen to palladium(II) is suppressed. Inorg. Chim. Acta 217, 21–28 (1994)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallick, S., Bera, B.K., Karmakar, P. et al. Kinetics and Mechanism of the Ligand Substitution Reaction of the Di-μ-hydroxobis(bipyridyl)dipalladium(II) Ion with some Bio-relevant Ligands. J Solution Chem 40, 532–544 (2011). https://doi.org/10.1007/s10953-011-9659-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-011-9659-5