Abstract
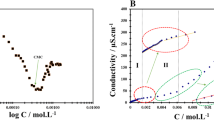
To understand the thermodynamic characteristics of cationic surfactants in binary mixtures, the aggregation behavior of hexadecyltrimethylammonium chloride (CTAC) has been investigated in ethylene glycol (EG) + water solvent mixtures at different temperatures and EG to water ratios. The critical micelle concentration (CMC) and degree of counter ion bonding (β) were calculated from electrical conductivity measurements. An equilibrium model for micelle formation was applied to obtain the thermodynamic parameters for micellization, including the standard Gibbs energies of micellization (\(\Delta G_{\mathrm{mic}}^{\mathrm{o}})\), standard enthalpies of micelle formation (\(\Delta H_{\mathrm{mic}}^{\mathrm{o}})\) and standard entropies of micellization (\(\Delta S_{\mathrm{mic}}^{\mathrm{o}})\). Our results show that \(\Delta G_{\mathrm{mic}}^{\mathrm{o}}\) is always negative and slightly dependent on temperature. The process of micellization is entropy driven in pure water, whereas in EG + water mixtures the micellization is enthalpy driven.
Similar content being viewed by others
References
Abbott, L.N., MacKay, A.R.: Surfactant Applications. Curr. Opin Colloid Interface Sci. 4, 322–324 (1999)
Schramm, L.L.: Surfactants: Fundamentals and Applications. In: The Petroleum Industry, pp. 3–22. Cambridge University Press, Cambridge (2000)
Galan, J.J., Gonzalez-Perez, A., Del Castillo, J.L., Rodriguez, J.R.: Thermal parameters associated to micellization of dodecylpyridinium bromide and chloride in aqueous solution. J. Therm. Anal. Calorim. 70, 229–234 (2002)
Perger, T.-M., Bester-Rogac, M.: Thermodynamics of micelle formation of alkyltrimethylammonium chlorides from high performance electric conductivity measurements. J. Colloid Interface Sci. 313, 288–295 (2007)
Akhter, M.S., Alawi, S.M.: Aggregation of ionic surfactants in formamide. J. Colloids Surf. A, Physicochem. Eng. Aspects 173, 95–100 (2000)
Akisada, H., Kuwahara, J., Noyori, K.: Critical micelle concentrations and interaction parameters of aqueous binary surfactant: ionic surfactant mixtures. J. Colloid Interface Sci. 288, 238–246 (2005)
Nagarajan, R., Wang, C.-C.: Solution behavior of surfactant in ethylene glycol: Probing the existence of a CMC and of micellar aggregates. J. Colloid Interface Sci. 178, 471–482 (1996)
Mehta, S.K., Chaudhary, S., Bhasin, K.K.: Spectral characterization and colloidal properties of 1-hexadecylpyridinium chloride in aqueous binary mixtures of different glycols. J. Colloid Interface Sci. 333, 646–654 (2009)
Chen, L.J., Lin, S.Y., Huang, C.C: Effect of hydrophobic chain length of surfactants on enthalpy-entropy compensation of micellization. J. Phys. Chem. B 102, 4350–4356 (1998)
Evans, D.F., Miller, D.D.: In: Friberg S.E., Lindman, B. (eds.) Organized Solutions. Surfactants in Science and Technology, pp. 33–45. Dekker, New York (1992)
Carnero Ruiz, C.: Thermodynamics of micellization of tetradecyltrimethylammonium bromide in ethylene glycol-water binary mixture. J. Colloid Polym. Sci. 277, 701–707 (1999)
Sugihara, G., Era, Y., Funatsu, M., Kunitake, T., Lee, S., Sasaki, Y.: Micelle formation of dodecylammonium surfactant with mixed counterions: perfluorocarboxylate and alkanesulfonate ions. J. Colloid Interface Sci. 187, 435–442 (1997)
Fujiwara, M., Okano, T., Nakashima, T.H., Nakamura, A.A., Sugihara, G.: A temperature study on critical micellization concentration (CMC), solubility, and degree of counterion binding of α-sulfonatomyristic acid methyl ester in water by electroconductivity measurements. J. Colloid Polym. Sci. 275, 474–479 (1997)
Aguiar, J., Molina-Bolivar, J.A., Peula-Garcia, J.M., Carnero Ruiz, C.: Thermodynamics and micellar properties of tetradecyltrimethylammonium bromide in formamide-water mixtures. J. Colloid Interface Sci. 255, 382–390 (2002)
Sadeghi, R., Hosseini, R.: Thermodynamic properties of surfactant sodium n-heptyl sulfonate in water and in aqueous solutions of poly(ethylene glycol) at different temperatures. J. Colloids Surf. A, Physicochem. Eng. Asp. 348, 177–185 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, J., Wang, D., Bu, F. et al. Investigation of the Thermodynamic Properties of the Cationic Surfactant CTAC in EG + Water Binary Mixtures. J Solution Chem 39, 1501–1508 (2010). https://doi.org/10.1007/s10953-010-9551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-010-9551-8