Abstract
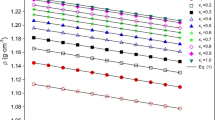
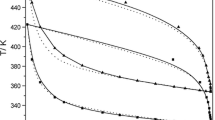
In this paper, the temperature and composition dependencies of the volumetric behavior are studied for the ethane-1,2-diol + 2-methoxyethanol + 1,2-dimethoxyethane + water quaternary system. Density data were collected at different temperatures ranging from −10 to 80 ∘C and at atmospheric pressure over the whole composition range, 0≤ x i (i = 1, 2, 3, 4)≤ 1. Moreover, we also made use of the results on the six binary (ij) and four ternary (kij) subsystems studied previously. The excess molar volume (VE) data have been fitted to an equation derived from the well-known Redlich–Kister equation and some interesting correlations were found. Furthermore, in order to represent in an effective way the behavior of the VE = VE(x i ) function (and of the derived partial molar quantities) in the quaternary domain, a new algorithm has been developed, which gives 3D plots where the dependent function is depicted by means of “colored slices” of the tetrahedron corresponding to the investigated composition quaternary domain.
Similar content being viewed by others
References
A. Marchetti, A. Martignani, and L. Tassi, Densities and Excess Molar Volumes of the Solvent (Ethane-1,2-diol + 2-Methoxyethanol + Water) from T = 263.15 K to 353.15 K, J. Chem. Thermodyn. 30, 653–669 (1998), and references therein.
P. Baraldi, G. C. Franchini, A. Marchetti, G. Sanna, L. Tassi, A. Ulrici, and G. Vaccari, Density and Volume Properties of Ethane-1,2-diol + 1,2-Dimethoxyethane + Water Ternary Mixtures from −10 to 80 °C, J. Solution Chem. 29, 489–504 (2000), and references therein.
M. Cocchi, A. Marchetti, L. Pigani, L. Tassi, A. Ulrici, G. Vaccari, and C. Zanardi, Density and Volume Properties of the 2-Methoxyethanol + 1,2-Dimethoxyethane + Water Ternary Solvent System at Various Temperatures, Phys. Chem. Liq. 39, 151–168 (2001), and references therein.
M. Cocchi, M. Manfredini, A. Marchetti, S. Sighinolfi, L. Tassi, A. Ulrici, and M. Vignali, The Ethane-1,2-diol + 2-Methoxyethanol + 1,2-Dimethoxyethane Ternary Solvent System: Density and Volume Properties at Different Temperatures, Phys. Chem. Liq. 39, 481–498 (2001), and references therein.
A. Paar, Digital Density Meter, Instruction Manual (Graz, Austria, 1984).
M. Cocchi, A. Marchetti, L. Pigani, G. Sanna, L. Tassi, A. Ulrici, G. Vaccari, and C. Zanardi, Density and Volumetric Properties of Ethane-1,2-diol + Di-ethylen-glycol Mixtures at Different Temperatures, Fluid Phase Equilib. 172, 93–104 (2000).
B. H. Hall, Ed., Time Series Processor—TSP—User’s Guide (TSP International, Stanford, CA, 1987).
J. A. Riddick, W. B. Bunger, and T. K. Sakano, Techniques of Organic Chemistry, 4th ed. (Wiley-Interscience, New York, 1986).
O. Chiavone-Filho, P. Proust, and P. Rasmussen, Vapor–Liquid Equilibria for Glycol Ether + Water Systems, J. Chem. Eng. Data 38, 128–131 (1993).
A. Sahgal and W. Hayduk, Ethylene Solubility and Diffusivity in Hexane–Dodecane and Ethylene Glycol–Butanol Solutions, J. Chem. Eng. Data 24, 222–227 (1979).
G. Douheret and A. Pal, Dielectric Constants and Densities of Aqueous Mixtures of 2-Alkoxyethanols at 25°C, J. Chem. Eng. Data 33, 40–43 (1988).
M. Dizechi and E. Marschall, Viscosity of Some Binary and Ternary Liquid Mixtures, J. Chem. Eng. Data 27, 358–363 (1982).
D. Bohne, S. Fischer, and E. Obermeier, Thermal Conductivity, Density, Viscosity, and Prandtl-Numbers of Ethylene Glycol–Water Mixtures, Ber. Bunsen-Ges. Phys. Chem. 88, 739–742 (1984).
C. M. Kinart, W. J. Kinart, and A. C. Wiklinska, Density and Viscosity at Various Temperatures for 2-Methoxyethanol + Acetone Mixtures, J. Chem. Eng. Data 47, 76–78 (2002).
R. Francesconi, C. Castellari, and F. Comelli, Excess Molar Enthalpies and Excess Molar Volumes of Diethyl Carbonate + Some n-Alkoxyethanols at (298.15 and 313.15) K, J. Chem. Eng. Data 44, 1373–1378 (1999).
A. Pal and S. Sharma, Excess Molar Volumes and Viscosities of Binary Liquid Mixtures of Ethylene Glycol Dimethyl Ether + Ethylene Glycol Monomethyl, + Diethylene Glycol Monomethyl, and + Triethylene Glycol Monomethyl Ethers at 298.15 and 308.15 K, J. Chem. Eng. Data 44, 212–215 (1999).
P. J. Victor and D. K. Hazra, Excess Molar Volumes, Viscosity Deviations, and Isentropic Compressibility Changes in Binary Mixtures of N-Methylacetamide + 2-Methoxyethanol and N-Methylacetamide + Water at (308.15, 313.15, and 318.15) K, J. Chem. Eng. Data 47, 79–82 (2002).
M. Nakata and M. Sakurai, Refractive Index and Excess Volume for Binary Liquid Mixtures. Part 1. Analysis of New and Old Data for Binary Mixtures, J. Chem. Soc., Faraday Trans. 1 83, 2449–2457 (1987).
K. Aizawa and M. Kato, Vapour Liquid Equilibrium Determination by Total Pressure Measurements for Three Binary Systems Made of 1,2-Dimethoxyethane with Toluene, Methylcyclohexane, or (Trifluoromethyl)benzene, J. Chem. Eng. Data 36, 159–161 (1991).
J. Barthel, R. Neueder, and H. Roch, Density, Relative Permittivity, and Viscosity of Propylene Carbonate + Dimethoxyethane Mixtures from 25 °C to 125 °C, J. Chem. Eng. Data 45, 1007–1011 (2000).
J. L. Cabezas, S. Beltran, and J. Coca, Isobaric Vapor–Liquid Equilibrium Data for the Binary Systems 1,2-Dimethoxyethane + Alcohols, J. Chem. Eng. Data 36, 184–188 (1991).
V. Viti and P. Zampetti, Dielectric Properties of 2-Methoxyethanol and 1,2-Dimethoxyethane: Comparison with Ethylene Glycol, Chem. Phys. 2, 233–238 (1973).
R. C. Weast, Ed., Handbook of Chemistry and Physics, 66th edn. (CRC, Boca Raton, FL, 1985).
N. Radojkovic, A. Tasic, D. Grozdanic, B. Djordevic, and M. M. Malic, Excess Volumes of Acetone + Benzene, Acetone + Cyclohexane, and Acetone + Benzene + Cyclohexane at 298.15 K, J. Chem. Thermodyn. 9, 349–356 (1977).
F. Kohler, Estimation of the Thermodynamic Data for a Ternary System from the Corresponding Binary Systems, Monatsch. Chem. 91, 738–740 (1960).
W. E. Acree Jr., Thermodynamic Properties of Nonelectrolyte Solutions (Academic Press, New York, 1984).
R. K. Jacob and K. Fitzner, The Estimation of the Thermodynamic Properties of Ternary Alloys from Binary Data Using the Shortest Distance Composition Path, Thermochim. Acta 18, 197–206 (1977).
I. Cibulka, Estimation of Excess Volume and Density of Ternary Liquid Mixtures of Non-Electrolytes from Binary Data, Coll. Czech. Chem. Commun. 47, 1414–1419 (1982).
I. Nagata and K. Tamura, Excess Molar Enthalpies for the Methanol–1-Butanol–Benzene System at 25°C, J. Chem. Eng. Data 33, 283–285 (1988).
M. Cocchi, M. Manfredini, A. Marchetti, R. Seeber, L. Tassi, and A. Ulrici, Mass Transport Properties in Solvent Liquid Mixtures, in Trends in Chemical Engineering, Vol. 7, R. Richard, Eds. Research Trends, (Trivandrum, India, 2001), pp. 47–79.
G. C. Pedrosa, J. A. Salas, and M. Katz, Excess Molar Volumes and Excess Viscosities of the n-Pentanol–Cumene–1,4-Dioxane System at 298.15 K, Thermochim. Acta 160, 243–252 (1990).
E. Caner, G. C. Pedrosa, and M. Katz, Excess Molar Volumes, Excess Viscosities and Refractive Indices of a Quaternary Liquid Mixture at 298.15 K, J. Mol. Liq. 68, 107–125 (1996).
O. Redlich and A. T. Kister, Thermodynamics of Nonelectrolyte Solutions—x–y–t Relations in a Binary System, Ind. Eng. Chem. 40, 341–345 (1948).
R. Lumry, E. Battistel, and C. Jolicoeur, Geometric Relaxation in Water. Its Role in Hydrophobic Hydration, Faraday Symp. Chem. Soc. 17, 93–108 (1982).
M. I. Davis, Thermodynamic and Related Studies of Amphiphile + Water Systems, Chem. Soc. Rev. 22, 127–134 (1993).
C. Tanford, The Hydrofobic Effect (Wiley, New York, 1980).
M. V. Ramanamurti, P. V. S. S. Prabhu, and S. L. Bahadur, Microheterogeneity in Aqueous Mixtures of Hydrophobic Solutes Through Viscosity Studies, Bull. Chem. Soc. Jpn. 59, 2341–2345 (1986).
J. Y. Huot, E. Battistel, R. Lumry, G. Villeneuve, J. F. Lavalle, A. Anusiem, and C. Jolicoeur, A Comprehensive Thermodynamic Investigation of Water–Ethylene Glycol Mixtures at 5, 25, and 45 °C, J. Solution Chem. 17, 601–636 (1988).
H. Eyring and M. S. John, Significant Liquid Structure (Wiley, New York, 1969).
W. Caminati and G. Corbelli, Conformation of Ethylene Glycol from the Rotational Spectra of the Nontunneling O-Monodeuterated Species, J. Mol. Spectrosc. 90, 572–578 (1981).
L. P. Kuhn and A. R. Wires, The Hydrogen Bond. VI. Equilibrium Between Hydrogen Bonded and Nonbonded Conformation of α,ω-Diol Monomethyl Ethers, J. Am. Chem. Soc. 86, 2161–2165 (1964).
T. P. Straatsma and J. A. McCammon, Treatment of Rotational Isomers in Free Energy Evaluations. Analysis of the Evaluation of Free Energy Differences by Molecular Dynamics Simulations of Systems with Rotational Isomeric States, J. Chem. Phys. 90, 3300–3304 (1989).
J. M. Resa, C. Gonzales, J. M. Goenaga, and M. Iglesias, Temperature Dependence of Excess Molar Volumes of Ethanol + Water + Ethyl Acetate, J. Solution Chem. 33, 169–198 (2004).
Y. Koga and P. Westh, Partial Molar Fluctuations in Aqueous 2-Butoxyethanol, Bull. Chem. Soc. Jpn. 69, 1505–1508 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulrici, A., Cocchi, M., Foca, G. et al. Study of the Dependence on Temperature and Composition of the Volumic Properties of Ethane-1,2-diol + 2-Methoxyethanol + 1,2-Dimethoxyethane + Water Solvent System and Graphical Representation in the Quaternary Domain. J Solution Chem 35, 139–159 (2006). https://doi.org/10.1007/s10953-006-9287-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10953-006-9287-7