Abstract
Polyethyleneimine (PEI) with an amount of –NH2 groups used in precursor solution could effectively reduce Cu2+ volatilization during the pyrolysis process. Thermogravimetric analysis shows that the temperature window of low-temperature pyrolysis for precursor solution with PEI (PEI-YBCO) is widened significantly. The slower pyrolysis process can enrich Cu2+ and improve critical current density (Jc) of PEI-YBCO films. The highest Jc is 3.03 MA/cm2 at 77 K when the amount of PEI is 0.5 g/10 mL and the film thickness is 400 nm. Then the thickness increases from 0.4 to 2.0 μm by changing the coating times. The Jc values of PEI-YBCO films decrease gradually with the thickness increase. However, the critical current (Ic) can be up to 197 A/cm (at 77 K, self-field) and Jc can still keep 1.68 MA/cm2 at 1.2 μm.
Similar content being viewed by others
1 Introduction
Second-generation high-temperature superconducting wires based on the REBa2Cu3O7−δ (REBCO, RE = Y, Gd, Sm, Dy, etc.)-coated conductors (CCs) have become a hot topic in magnetic fields. In order to introduce YBa2Cu3O7−δ superconductors into commercial application, a metalorganic decomposition (MOD) method has been established as the versatile method to grow low-cost, scalable, high-performance epitaxial YBa2Cu3O7 (YBCO) films for coated conductors [1,2,3,4,5]. To ensure the high critical current (Ic) of obtained films, thick YBCO films were required. However, with the thickness increase, thickness effect and rapid volatilization of copper ions could tremendously degrade the superconducting properties of the obtained MOD-YBCO films. To overcome these two problems, several possible approaches were investigated, such as doping with rareearth metal [6], doping with secondary phases [7] and coating multilayers with ultrathin interlayers [8,9,10]. In addition, evidences verified that polymers facilitate the control of the precursor viscosity and bind metal ions, which are advantageous to form homogeneous precursor solution and further form uniform films [11]. Therefore, partial researchers tried to modify precursor solution by adding different organic additives to improve the surface morphology and superconducting properties of YBCO films including diethanolamine (DEA) [12], polyvinyl alcohol (PVA) [13], diacetone acrylamide (DAAM) [6] and acetylacetone (ACAC) [14]. Regrettably, no research has been reported that significantly overcome these two difficulties simultaneously. Taking into account these factors, we introduce an effective way to weaken thickness effect and reduce the volatilization of copper ions by adding polyethyleneimine (PEI) in precursor solution. A large number of electron-donating –NH2 groups in PEI can coordinate with Cu ions to form much more stable chelates, which could help enrich Cu ions in final YBCO films. It is worth noting that the chelates contain abundant hydrogen bonds to widen a temperature window of the precursor solution decomposition process, which favor the stability of the sintering process and thick film structure. The critical current (Ic) can be up to 197 A/cm (at 77 K, self-field) and high Jc (0 T, 77 K) of over 1.68 MA/cm2 was obtained at 1.2 μ m.
2 Experimental
2.1 Precursor Solution Preparation
The YBCO precursor solutions were prepared by mixing three solutions, named as Y-solution, Ba-solution and Cu-solution. Stoichiometric quantities of yttrium and copper acetates were dissolved in methanol with an excess of propionic acid to obtain the Y-solution and Cu-solution. The Ba-solution was prepared by dissolving barium acetate in methanol with an excess of trifluoroacetic acid. The three solutions were distilled separately to remove a small amount of water and other impurities, then the three solutions were mixed together corresponding to the 1:2:3.15 stoichiometry and the distillation was continued under vacuum. After repeating the refining process several times, dark green gel with organic solvent was obtained (pure YBCO solution). The total metal ion concentration was 1.9 mol/L. PEI (branched PEI, Sigma-Aldrich Co. LLC; Fluka 50% (w/v) in 100 mL H2O) was added to pure YBCO solution to obtain another stable greencolored solution (PEI-YBCO solution). The PEI is a high molecular polymer, and its specific molecular weight cannot be determined. In this experiment, we adjusted the amount of added mass concentration to get the optimum amount. For YBCO, six such portions were taken and provided with PEI amounts leading to the following PEI/10 mL ratios: 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 g/10 mL, respectively.
2.2 Coating Process
The coating process parameters of monolayer and multilayer films is the same: the speed of dip coating was 500 μ m/s, and the temperature and relative humidity were controlled at 20 ∘C and 30%, respectively. The PEI-YBCO and pure YBCO precursor solutions were deposited on SrTiO3 (STO) singlecrystal substrates by a dip-coating process to obtain single-layer PEI-YBCO (1-PEI) and pure YBCO (1-pure) films. The three-layer (3-PEI) and five-layer (5-PEI) PEI-YBCO pyrolytic films were obtained by the repeated three or five times coating-pyrolysis process on STO singlecrystal substrates. Then the pyrolytic film was annealed at 780 ∘C to obtain a multilayer superconducting film (3-PEI, 5-PEI).
2.3 Heat Treatment Process
Heat treatment has been divided into two parts: pyrolysis and crystallization/oxygenation. Wet gel films were firstly dried in dry O2 atmosphere, then pyrolyzed in humid O2 by heating them to 400 ∘C with 5 ∘C/min and kept 0.5 h at 400 ∘C to ensure that decomposition was complete. During the second part the films were pyrolyzed in a humid Ar/O2 and held at 550 ∘C for 30 min to obtain the amorphous matrix with nanocrystalline materials of Y, Ba, and Cu oxides. In the annealing process, samples were sintered at 780 ∘C in a humid and dry Ar/O2 for 120 and 30 min, respectively (oxygen partial pressure was 150 ppm). Oxygenation followed in dry O2 at 450 ∘C for about 1 h with subsequent furnace cooling of the samples.
2.4 Analytical Instruments
Thermogravimetric analysis (TGA) was performed with a Netzsch STA 449C thermal analyzer at a heating rate of 5 ∘C/min in the air. Phase composition and crystal structure of YBCO films were analyzed by 𝜃–2𝜃 X-ray diffraction (XRD). The critical current density (Jc) of YBCO films was examined by inductive measurement using a Cryoscan by Theva. The EDS mapping and microstructure of the films were observed by scanning electron microscope (JSM7500F).
3 Results and Discussion
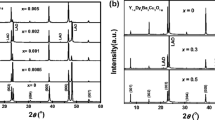
XRD patterns of the YBCO films with different contents of PEI are shown in Fig. 1a. When the content of PEI was 0∼0.5 g/10 mL, the strong reflections of YBCO (00l) films were observed, which indicated good c-axis texture. Disappointingly, a large number of miscellaneous peaks appeared with the content of PEI increased to 0.6 g/10 mL Fig. 1b shows that the Jc of YBCO films dropped with the addition of PEI, increasing rapidly to 3.03 MA/cm2 (77 K, 0 T) with the 400 nm film thickness and 0.5 g/10 mL content of PEI, which was chosen for further investigations.
In order to determine the impact of the PEI addition on thermal decomposition, thermal analysis of pure YBCO and PEI-YBCO solutions was performed by TGA and DTA in air atmosphere with a 5 ∘C/min heating rate (Fig. 2). Up to 170 ∘C, both solutions shown very similar curve progressions with slight weight loss for both the TGA and the DTA signals, which were caused by the evaporation of unbound solvent. The second mass loss of pure YBCO solution happened at 198∼352 ∘C (mass loss ∼ 52.48%) and exhibited one strong exothermic peak near 308 ∘C. And the mass loss of PEI-YBCO solution happened at 172∼367 ∘C (mass loss ∼ 58.9%) with three characteristic exothermic peaks at 265, 296 and 330 ∘C, respectively, which should be caused by salt pyrolysis [11]. Significantly, the temperature window became wider and the number of pyrolysis peaks became more than ever. The result pointed to a moderated behavior for the decomposition process in the low temperature range when PEI was added to precursor solutions, which was helpful to reduce the internal cavity, prevent the films from cracking and improve the quality of the films.
The UV spectra of copper acetate [Cu(CH3COO)2], copper propionate [Cu(CH3CH2COO)2] and Cu(CH3CH2COO)x(PEI)y solutions are shown in Fig. 3. Compare with the other two solutions, the absorption peak of the Cu(CH3CH2COO)x(PEI)y precursor solutions decreased between 200 and 400 nm, indicating a stronger chelating reaction which occurred after adding PEI As shown in the FTIR spectra of (CH3COO)2Cu⋅H2O (in Fig. 4), the peaks at 3475 cm− 1 correspond to the crystal water, and the bands at 1602 and 1445 cm− 1 represent COO− and –CH3, respectively. The IR spectrum of copper propionate is very similar to that of copper acetate, so it is not shown in the figure. For PEI, the characteristic absorption bands at 3279 cm− 1 (N–H stretch vibrational), 2128 cm− 1 (N–H bending), and 2900 cm− 1 (C–H stretch vibrational). When PEI was added, a appeared about 3118 cm− 1 and the peak at 2128 cm− 1 disappeared. It also indicates that PEI has a strong chelating reaction with Cu2+. Due to the existence of a large number of electron-donating group –NH2, PEI can react with Cu2+ to produce four coordination compounds [15]. The reaction equation is shown in Scheme 1. Depending on the amount of PEI added, Cu(CH3CH2COO)2 was progressively reassigned to two new species—the mixed ligand complex Cu(CH3CH2COO)x(PEI)y and the fully transformed Cu (PEI)z (whereby the latter was assumed to reach nearly 100% at very high PEI concentrations) in PEI-YBCO solution resulting in a slight change of the decomposition process in the low temperature range. Of course, we do not fully exclude PEI interacting with either Ba or RE as well, but Ba and RE were found to have difficulty in forming stable coordinated compounds with N respectively, so the main interactions are related to the Cu.
Generally, for the stoichiometry of metal ions in YBCO films, Y and Ba ions can hardly volatilize during the preparation of the sample, while Cu2+ is prone to volatilize in the process of pyrolysis for YBCO films. The imbalance of the proportion of metal ions in the film made it easy to form impurity phases [16], which affected the characteristic and performance of the films. Therefore, reducing the volatilization of copper ions is more advantageous to achieve a good stoichiometry. In order to investigate the elemental content, the EDS mapping of 1-PEI and 1-pure films was tested (Fig. 5). After hightemperature crystallization, the weight percentage of copper was 36.25% for 1-PEI film and 27.49% for 1-pure film. The result revealed that the addition of PEI could retain the copper to a large extent. According to the previous literature [17], Cu2+ and water-soluble PEI were used to quantitatively form four coordination complexes, and the process was very fast and the chelate was stable as well. Combined with the experimental result of EDS mapping, the addition of PEI can reduce the volatilization of copper ions in the heat treatment process and guarantee the ideal stoichiometry of metal ions in YBCO films.
On the basis of the study for single-layer films, PEI-YBCO (0.5 g/10 mL) precursor solution was used to prepare PEI-YBCO films with different thicknesses. The different thicknesses were achieved by coating different layers of the film after pyrolysis and finally crystallization 3-PEI and 5-PEI PEI-YBCO films were investigated in the following experiments. The textures, surface morphologies and properties of PEI-YBCO films with different thicknesses were characterized.
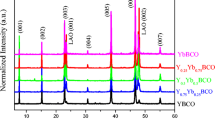
The XRD patterns of different layers of PEI-YBCO films (Fig. 6) illustrated that pure c-axisoriented growth of YBCO with 1.2 μ m thickness of the 3-PEI film. XRD patterns of the 5-PEI film (d = 2.0 μ m) showed a-axis grains, and the amount of a-axis grains was evaluated by the ratio of α = (200) / (006), and the value of α5−PEI was 0.148. Furthermore, it is hard to avoid a small amount of BaF2 and other impure phases (such as Ba-Cu-O) appeared in the 5-PEI film, which become more dominant in thicker films.
For further examining the texture, omega-scan and phi-scan rocking curves are shown in Fig. 7, indicating their good in-plane and out-plane texture. Although the FWHM values of PEI-YBCO films decreased continuously with the thickness increase, the texture of film still maintained for the 5-PEI film. This is one evidence that the PEI-YBCO films showed high properties in our work.
As shown in Fig. 8, no a-axis grains were observed on the surface of 1-PEI and 3-PEI films and a small amount of strip-like a-axis grains appeared on the surface of the 5PEI film. It confirms the c-oriented films which grow well for PEI-films, which was also conformed to the XRD results. On the one hand, this might prevent extending the segregation of copper species by condensation and thus maintain well-homogenized distribution of all metal cations within the film, which was crucial for the conversion into homogenous YBCO films. On the other hand, in the process of lowtemperature pyrolysis, PEI can form a large molecular network structure in the process of film growth. So the surface structure of the film was more compact. It can be inferred that the slowdown of the release of volatile species in PEI-YBCO precursor solutions could have positive effects on the microstructure in macroscopic scale.
The critical current density in self-field at 77 K was improved by PEI modification, as shown in Fig. 9. The Jc values decreased from 3.03 to 0.93 MA/cm2 as the number of coating layer was from 1 (d = 0.4 μ m) to 5 (d = 2.0 μ m). Nevertheless, the Ic increases first and then decreases with the thickness increase which is the same result as that shown by scanning electron microscopy (SEM) and XRD spectra, and a maximum Ic value of 197 A/cm was obtained on the 3-PEI film. The decrease in Ic of the 5-PEI film was mainly caused by the thickness effect of the film.
4 Conclusion
In summary, small additions of PEI in YBCO precursor solutions can react with Cu2+ to form Cu(PEI)z and Cu(CH3CH2COO)x(PEI)y to cause a very positive effect on the microstructural characteristics of YBCO films and, consequently, improve the critical current densities. The highest Jc of the PEI film can be achieved over 3.03 MA/cm2 (77 K, self-field) by adding 0.5 g PEI in 10 mL precursor solution. For the study of multilayer thick films, the FWHM values of PEI-YBCO films decreased as the thickness increased, as well as critical current densities. Nevertheless, the Ic increases at first and strongly up to 3-PEI with 197 A/cm then drops with the thickness increase by the thickness effect of the film.
References
Foltyn, S., Civale, L., MacManus-Driscoll, J., Jia, Q., Maiorov, B., Wang, H., et al.: Materials science challenges for high-temperature superconducting wire. Nat. Mater. 6, 631–642 (2007)
Obradors, X., Puig, T.: Coated conductors for power applications: Materials challenges. Supercond. Sci. Technol. 27, 044003 (2014)
Chen, D.-X., Pardo, E., Sanchez, A., Bartolome, E., Puig, T., Obradors, X.: Magnetic properties of a melt-textured YBa2Cu3 O x ring with a perpendicular crack. Appl. Phys. Lett. 90, 072501 (2007)
Shiohara, Y., Taneda, T., Yoshizumi, M.: Overview of materials and power applications of coated conductors project. Jpn. J. Appl. Phys. 51, 010007 (2011)
Palmer, X., Pop, C., Eloussifi, H., Villarejo, B., Roura, P., Farjas, J., et al.: Solution design for low-fluorine trifluoroacetate route to YBa2Cu3 O 7 films. Supercond. Sci. Technol. 29, 024002 (2015)
Nakaoka, K., Yoshizumi, M., Usui, Y., Izumi, T., Shiohara, Y.: Improvement of production rate of YBCO coated conductors fabricated by TFA-MOD method. Phys. Procedia 58, 134–137 (2014)
Maiorov, B., Baily, S., Zhou, H., Ugurlu, O., Kennison, J., Dowden, P., et al.: Synergetic combination of different types of defect to optimize pinning landscape using BaZrO3,-doped YBa2Cu3 O 7. Nat. Mater. 8, 398–404 (2009)
Foltyn, S., Wang, H., Civale, L., Jia, Q., Arendt, P., Maiorov, B., et al.: Overcoming the barrier to 1000 A/cm width superconducting coatings. Appl. Phys. Lett. 87, 162505 (2005)
Wuwei, Y., Chengxiao, Z., Guofang, Z., Lihua, J., Chengshan, L., Guo, Y., et al.: Preparation of YBCO superconducting film by chemical solution deposition using a novel copper precursor derived from PEG (200) and sucrose. Rare Metal Mater. Eng. 40, 346–349 (2011)
Xu, W.C., Bai, Y.L., Fang, J.H., Cai, C.B.: The effect of polyethyleneimine (PEI) on the texture and morphology of YBCO films. In: Advanced Materials Research, pp. 103–106 (2014)
Jia, QX, McCleskey, TM, Burrell, AK, et al.: Polymer-assisted deposition of metal-oxide films. Nat. Mater., 529–532 (2004)
Dawley, J., Clem, P., Boyle, T., Ottley, L., Overmyer, D., Siegal, M.: Rapid processing method for solution deposited YBa2Cu3 O 7−δ thin films. Physica C: Supercond. 402, 143–151 (2004)
Wang, W., Li, G., Pu, M., Sun, R., Zhou, H., Zhang, Y., et al.: Chemical solution deposition of YBCO thin film by different polymer additives. Physica C: Supercond. 468, 1563–1566 (2008)
Erbe, M., Hänisch, J., Freudenberg, T., Kirchner, A., Mönch, I., Kaskel, S., et al.: Improved REBa2Cu3 O 7−x(RE = Y, Gd) structure and superconducting properties by addition of acetylacetone in TFA-MOD precursor solutions. J. Mater. Chem. 2, 4932–4944 (2014)
Chen, Y., Pan, B., Li, H., Zhang, W., Lv, L., Wu, J.: Selective removal of Cu(II) ions by using cation-exchange resin-supported polyethyleneimine (PEI) nanoclusters. Environ. Sci. Technol. 44, 3508–3513 (2010)
Lee, J.-W., Joo, Y.-S., Choi, S.-M., Yoo, S.-I.: Enhanced flux pinning properties of MODprocessed YBa2Cu3O7-delta thin films with BaZrO3 nanoparticles using a Ba-deficient coating solution. IEEE Trans. Appl. Supercond. 23, 4 (2013)
An, F.-q., Gao, B.-j., Liu, Q.: Determining structure of chelate complex of polyethyleneimine with cupric ion and its analysis application. Chin. J. Anal. Labor. 25, 115 (2006)
Funding
This work was supported by the National Natural Science Foundation of China (21571126, 51572165, 11174193 and 51202141) the Shanghai Key Laboratory of High Temperature Superconductors (14DZ2260700) and the Science and Technology Commission of Shanghai Municipality (16521108400, 16DZ0504300 and 14521102800).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jing Wang and Xiuping Yin contributed to the work equally and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Wang, J., Yin, X., Bai, YL. et al. Improved Superconducting Properties of Thick YBa2Cu3O7−δ Film by Adding Amino-Rich Polyethyleneimine in Precursor Solution. J Supercond Nov Magn 31, 3503–3508 (2018). https://doi.org/10.1007/s10948-018-4642-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-018-4642-7