Abstract
Spinel nickel aluminate (NiAl 2 O 4) nanospheres (NSPs) and nanoplatelets (NPLs) were successfully synthesized using a facile microwave combustion method (MCM) and conventional combustion method (CCM), respectively, using urea as the fuel. The as-prepared samples were characterized by powder X-ray diffraction (XRD), Fourier transform infrared (FT-IR), high-resolution scanning electron microscopy (HR-SEM), high-resolution transmission electron microscopy (HR-TEM), energy-dispersive X-ray spectroscopy (EDX), selected area electron diffraction (SAED), UV–Visible diffuse reflectance spectroscopy (DRS), photoluminescence (PL) spectroscopy, and vibrating sample magnetometer (VSM) analysis. Powder XRD, FT-IR, EDX, and SAED results showed that the samples were pure phase spinel NiAl 2 O 4 nanocrystals without any other secondary phase impurity. UV–Vis DRS and PL spectroscopy results were used to calculate the energy band gap (E g) value of the samples which is 3.41 and 3.08 eV for NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs, respectively. VSM results of the NiAl 2 O 4 NPLs sample show lower M s (49.16 × 10 −3 emu/g) values compared with the NiAl 2 O 4 NSPs sample (73.42 × 10 −3 emu/g), which confirm that both the products have ferromagnetic behavior. NiAl 2 O 4 NSPs were found to have higher surface area than NiAl 2 O 4 NPLs, which in turn leads to the improved performance towards the selective oxidation of benzyl alcohol into benzaldehyde, with 100 % selectivity, and it was found that the NiAl 2 O 4 NSPs sample show higher conversion (96.25 %) than NiAl 2 O 4 NPLs (85.37 %). The as-prepared samples show high activity, good reusability, remarkable stability, and environmentally friendly materials for industrial and technological applications.

Spinel NiAl2O4-NSPs and NiAl2O4-NPLs samples were synthesized by MCM and CCM techniques respectively. Structural, morphological, magneto-optical and catalytic properties were studied systematically. The calculated optical band gap value of the sample NiAl2O4-NSPs is 3.41 eV, which is higher than NiAl2O4-NPLs (3.08 eV), due to the smaller particle size. The relatively lower Ms (49.16 × 10-3 emu/g) of NiAl2O4-NPLs shows that it is weak ferromagnetism and high Ms (73.42 × 10-3 emu/g) of NiAl2O4-NSPs confirms its ferromagnetic behavior. Catalytic oxidation of benzyl alcohol into benzaldehyde was found that the sample NiAl2O4-NSPs show higher conversion (96.25 %) than NiAl2O4-NPLs (85.37 %) with 100 % selectivity.
Similar content being viewed by others
1 Introduction
Nowadays, nanostructured spinel type mixed metal oxides have attracted significant attention in many areas such as ceramics, semiconductors, sensors and catalytic materials [1–6]. Among them, spinel aluminates have high thermal stability, hydrophobicity, high mechanical resistance, and low surface acidity [7–13]. Nanocrystalline spinel aluminates with the general formula AB 2 O 4, where A 2+ (tetrahedral) and B 3+ (octahedral) are divalent (Ni 2+, Cu 2+, Co 2+, etc.) and trivalent (Al 3+) cations, respectively, attract the research interest, because of their versatile practical applications. Over the spinel metal aluminates, nickel aluminate (NiAl 2 O 4) have been studied for many applications, including magnetic materials, pigments, catalysts, and refractory materials, which belong to the space group Fd3m [14, 15]. NiAl 2 O 4 nanostructures have unique properties such as structural, morphological, optical, electrical, magnetic and catalytic activities compared with bulk materials, due to their smaller particle size and higher surface area. Its remarkable electrical and magnetic properties depend upon the nature of the ion distribution among A and B sites [16]. NiAl 2 O 4 is an inverse spinel in which half of the Al 3+ ions preferentially fill the A sites and the other occupies the B sites. Thus, the spinel NiAl 2 O 4 can be represented by the formula (Al 3+)A(Ni 2+Al\(^{\mathrm {3+}})_{\mathrm {B}}\textit {O}_{\mathrm {4}}^{\mathrm {2-}}\) [17, 18].
It is well known that nanoparticles with controlled size and composition have fundamental and technological interest in nanoscience and nanotechnology. Spinel NiAl 2 O 4 nanostructures have been prepared using several methods, such as solid-state reaction, solgel, co-precipitation, hydrothermal solvothermal methods [19–24], etc. Spinel-type mixed metal oxides are usually prepared using a ceramic technique, which involves high-temperature solid-state reactions that resulted to large and non-uniform size particles [25]. Nowadays, many researchers have been used the combustion synthesis for the preparation of a variety of advanced ceramics, catalysts and nanomaterials. Moreover, microwave combustion method (MCM) offers great advantages such as prompting the rapid reaction due to the molecular level interaction of the microwave radiations with the reactant species. Also, MCM route is an easy and convenient one than the above mentioned methods. Moreover, it produces the homogeneous distribution of nanostructures at lower temperatures without further calcinations and also shorter reaction times, when compared with the above methods [26].
The advantage of the MCM technique is the dispersion of the raw materials in liquid precursors, which produced the crystallized nanopowders with smaller particle size and high-purity products with large scale [27]. It is a clean, cheap, and convenient route, which results in uniform particle morphology and shorter reaction time and is also a promising method for the preparation of various nanostructured materials with different sizes and shapes. Moreover, it is environmentally friendly, simple to operate and energy efficient over the conventional methods [28], due to the yields of the final products of metal oxides associated with their special structural personalities, namely nano-sized dimensions. Besides, to our knowledge, no literature is available on the magneto-optical properties of spinel NiAl 2 O 4 nanostructures using a simple microwave combustion method using urea as the fuel for the applications of catalytic oxidation of benzyl alcohol into benzaldehyde.
Spinel NiAl 2 O 4 nanostructures have received much attention as a catalyst, due to its stability and reusability, and it can be recovered easily from the reaction mixture using a simple filtration method [29]. It is well known that the catalytic activity of the ceramic materials depends on grain size and surface area, making it suitable for catalyst support [30]. Moreover, spinel NiAl 2 O 4 is non-toxic, inexpensive, and relatively high surface area making them fit for use as a cost-effectively and eco-friendly viable solid heterogeneous catalyst. The as-prepared spinel NiAl 2 O 4 nanostructures were investigated for structural, morphological, optical, magnetic, and catalytic properties, and different characterizations have been carried out using powder X-ray diffraction (XRD), Fourier transform infrared (FT-IR), high-resolution scanning electron microscopy (HR-SEM), high-resolution transmission electron microscopy (HR-TEM), energy-dispersive X-ray spectroscopy (EDX), Brunauer–Emmett–Teller (BET), UV–Visible diffuse reflectance spectroscopy (DRS), photoluminescence (PL) spectroscopy, and vibrating sample magnetometer (VSM) techniques, and the obtained results are presented here.
2 Experimental Part
2.1 Preparation of Spinel NiAl 2 O 4 Nanostructures Using Microwave Combustion Method and Conventional Combustion Method
All the chemicals used in this study were of analytical grade obtained from Merck, India, and were used as received without further purification. All chemicals such as nitrates of nickel and aluminum and urea were used as the fuel for this method. The stoichiometry of metal nitrate salts and fuel was calculated based on the total oxidizing and reducing valences related to the oxidant agents and to the fuels. Initially, the precursor mixture of nitrate solution in urea was placed in a domestic microwave oven and exposed to the microwave energy in a 2.45-GHz multimode cavity at 850 W for 10 min. When the solution attained the point of spontaneous combustion, ignition took place, resulting in a rapid flame and yielding solid fluffy final products. The obtained solid powders were washed well with water and ethanol several times and dried at 80 ∘C for 1 h and labeled as NiAl 2 O 4 nanospheres (NSPs) and then used for further characterizations.
In the next separate experiment, conventional combustion method (CCM), the same reaction mixture was taken in the silica crucible and treated in an air furnace at 500 ∘C for 2 h at a heating rate of 5 ∘C/min and cooled at the same rate; it became solid, which was labeled as NiAl 2 O 4 nanoplatelets (NPLs).
2.2 Characterizations of Spinel NiAl 2 O 4 Nanostructures
The structural characterization of spinel NiAl 2 O 4 nanostructures was performed using a Philips X’Pert X-ray diffractometer with CuK α radiation at λ = 1.540 Å. The surface functional groups were analyzed using a PerkinElmer FT-IR spectrometer. Morphological studies and energy-dispersive X-ray analysis of spinel NiAl 2 O 4 samples have been performed with a JEOL JSM6360 high-resolution scanning electron microscope. The transmission electron micrographs were carried out by Philips CM20 TEM. The surface area was derived from the N 2 adsorption–desorption isotherms using liquid nitrogen at 77 K using an automatic adsorption instrument (Nova 1000 gas sorption analyzer; Quantachrome Corp.). The UV–visible diffuse reflectance spectrum of spinel NiAl 2 O 4 samples was recorded using a Cary 100 UV–visible spectrophotometer to estimate their energy band gap (E g). The photoluminescence properties were recorded at room temperature using a Varian Cary Eclipse Fluorescence Spectrophotometer. The magnetic properties were investigated at room temperature in an applied magnetic field sweeping from −10 to + 10 KOe using a PMC MicroMag 3900 model vibrating sample magnetometer (VSM) equipped with a 1-T magnet.
2.3 Catalytic Test
The oxidation of benzyl alcohol using spinel NiAl 2 O 4 nanocatalysts was carried out in a batch reactor operated under atmospheric conditions. Five micromolars of the oxidant (H 2 O 2) was added along with 0.5 g of nano-sized NiAl 2 O 4 catalysts (NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs), and the contents were heated at 80 ∘C in an acetonitrile medium for 5 h in a three-neck round-bottom flask equipped with a reflux condenser and thermometer. The oxidized products after the catalytic reaction are collected and studied using an Agilent GC spectrometer. The column used for the study was DB wax column (capillary column) with a length of 30 mm, and helium was used as the carrier gas. GC technique was carried out to know the conversion percentage of the products.
3 Results and Discussion
3.1 Scanning Electron Microscopy Studies
The surface morphology of the samples was investigated by high resolution scanning electron microscopy (HR-SEM) analysis. The HR-SEM images of NiAl 2 O 4 nanospheres (NSPs) prepared using the MCM route are shown in Fig. 1a, b, which confirm the presence of NSPs like particle morphology with agglomeration. Therefore, we could infer that the spinel NiAl 2 O 4 NSPs was formed in the MCM route. It reveals that the NSPs are homogeneous and grains are distributed uniformly with smaller size and large agglomerates with a diameter below 10 nm, due to the fact that the MCM route is a volumetric and uniform heating method, when compared to the CCM route, which is a non-uniform and radiative method. Within the limited time, nucleation and growth had to be finished using the MCM route; hence, the particles with smaller and narrow distribution were obtained.
Figure 1c, d shows the HR-SEM images of NiAl 2 O 4 NPLs prepared using the CCM route, which confirm the formation of nanoplatelets (NPLs) like morphology with agglomeration. In the CCM, the reaction was completed only after 2 h, whereas in the MCM, the reaction was completed within few minutes of time. However, in the CCM, the time might be sufficient to form the separate phases, which has led to the bigger particle size of 20 nm with wider distribution, but in the MCM route, the size of the particle is only below 10 nm. Therefore, we can infer that the agglomerated NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs formed during the MCM and CCM approaches, respectively. Thus, the higher temperature and non-uniform heating method of CCM reaction in a furnace caused the grain growth than the MCM route. Moreover, the NiAl 2 O 4 NPLs sample is exposed for more time, and it results in the formation of agglomeration (may be due to the sintering effect). However, the morphology of the final products depends on the preparation methods and temperatures.
3.2 Transmission Electron Microscopy Studies
In order to further confirm the morphology and particle size of the samples, high resolution transmission electron microscopy (HR-TEM) studies are carried out. Figure 2a shows the HR-TEM image of the spinel NiAl 2 O 4 sample prepared using the MCM route, which confirms the formation of NSPs like particle morphology with smaller size. The obtained particles are nearly spherical in shape with a uniform size distribution for the NiAl 2 O 4 NSPs sample. The average size and void space diameter of single NiAl 2 O 4 NSPs are found to be in the range of 7–10 nm. It may be due to the fact that spinel NiAl 2 O 4 was prepared within a short reaction time of 10 min by means of a domestic microwave oven operated at 2.45 GHz (850 W).
The image of spinel NiAl 2 O 4 NPLs like particles with bigger particle size for the NiAl 2 O 4 NPLs sample prepared using the CCM route is shown in Fig. 2b, which confirms the formation of NPLs like particle morphology with a diameter range of 18–22 nm in size. Moreover, the nanoparticles prepared under MCM route were of narrower distribution than those prepared using the CCM approach. It is concluded that the temperature is a key factor in the controlled synthesis of nano-sized materials. Herein, spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs like particles were prepared by the temperature optimization only and there is no use of any other solvent, surfactant, and catalyst. Also, the mean particle size determined by HR-TEM analysis is very close to the average crystallite size calculated using the Scherrer formula from the powder X-ray diffraction (XRD) pattern, which is reported later in Section 3.4. The crystallinity and crystalline nature of the samples were confirmed by selected area electron diffraction (SAED) pattern. The SAED pattern of the spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs is shown in Fig. 2a, b (insets), respectively. The SAED pattern implies that the prepared samples are good crystalline materials with higher crystalline nature.
3.3 Energy-Dispersive X-ray Analysis
The purity and elemental compositions of the samples were confirmed by means of energy dispersive X-ray spectroscopy (EDX) analysis. Figure 3a b shows the EDX spectra of NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs, respectively. It exhibited Ni, Al, and O peaks and indicated the presence of NiAl 2 O 4 phase without any other impurity. In the EDX pattern, the presence of Al, Ni, and O elements in proper proportions suggested that the expected stoichiometry was maintained in the prepared samples. It is in good agreement with the powder XRD analysis, which is reported later in Section 3.4. The peak at 2.1 KeV in the EDX spectra were due to the gold coated samples before HR-SEM recording for better visibility of the surface morphology
3.4 Powder X-ray Diffraction Analysis
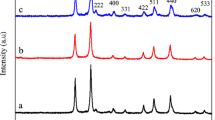
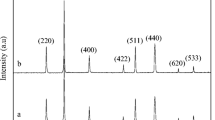
The powder XRD pattern of the prepared samples is given in Fig. 4. Figure 4a b shows the powder XRD pattern of the NiAl 2 O 4 NSPs (prepared using the MCM) and NiAl 2 O 4 NPLs (prepared using the CCM) samples respectively using urea as the fuel It revealed that both the NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples had higher crystalline nature The diffraction peaks at 2 𝜃 values of 31.27, 37.04, 38.87, 45.06, 49.26, 55.73, 59.38, 65.43, 74.28 and 77.51 correspond to (220), (311), (222), (400), (331), (422), (511), (440), (620), and (533) planes, respectively, which can be readily assigned to a cubic phase of spinel NiAl 2 O 4 (space group Fd3m). The diffraction peaks of the samples matched well with the JCPDS card no. 10-0339 [31]. No characteristic peak related to any other secondary phase impurity was observed. Further observation revealed that both the samples had sharp peaks indicating good crystallinity, but the diffraction peaks of the NiAl 2 O 4 NSPs sample were slightly broadened compared with NiAl 2 O 4 NPLs due to the smaller crystallite size. The average crystallite size for both the samples was calculated using the Scherrer formula.
where L is the average crystallite size (nm), λ is the X-ray wavelength (0.154 nm), 𝜃 is the diffraction angle and β is the full width at half maximum of the observed peaks. The average crystallite size for both the NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples was found to be 7.92 and 21.45 nm, respectively. The average crystallite size of the NiAl 2 O 4 NSPs sample is lower than that of the NiAl 2 O 4 NPLs sample, which are calculated from the full width at half maximum of the (311) plane. The observed higher crystallite size of NiAl 2 O 4 NPLs (prepared using the CCM route) is due to the high-temperature calcinations (500 ∘C for 2 h) compared with the NiAl 2 O 4 NSPs sample prepared using the MCM route [32]. However, the MCM approach produces spinel NiAl 2 O 4 NSPs in a microwave oven operated at a power of 850 W and a temperature ranging from 150 to 400 ∘C that would result to the formation of final products within 10 min with smaller crystallite size. Compared with the CCM, MCM is more time saving and higher in production scale. Also, the broad reflection peaks of the NiAl 2 O 4 NSPs sample proved very fine crystallite size prepared using the MCM route. However, when heat treatment in the CCM route was increased, the diffraction lines become narrower and more intense, resulting in higher crystallite size (NiAl 2 O 4 NPLs), which can be further understood by heat treatment at higher temperature (500 ∘C) to enhance the crystallite size of spinel NiAl 2 O 4 [33].
3.5 Fourier Transform Infrared Analysis
The Fourier transform infrared (FT-IR) spectral analysis has been used to study the functional groups of the samples. Figure 5a and b shows the FT-IR spectra of NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples prepared using both CCM and MCM, respectively and exhibits a broad band near 3450 cm −1, due to the –OH stretching vibration, and a second typical absorption band at 1612 cm −1 is assigned to the deformative vibration of H 2O molecules, which is most probably, due to the H 2O absorption when making the samples by pellets with KBr [34]. The metal–oxygen (Ni–O, Al–O and Ni–O–Al) stretching frequencies were observed in the range 450–650 cm −1, which are associated with the vibrations of Al–O, Ni–O and Ni–O–Al bonds. The characteristic of metal–oxygen frequencies present in the molecule indicates the formation of single-phase spinel NiAl 2 O 4 [35]. The absorption at 1452 and 1124 cm −1 corresponds to the vibration mode of Al–OH and Ni–OH, which are typical of this class of materials. In addition, a strong band at 1745 cm −1 is essential due to OH– vibration modes of hydrogen bonded –OH. No other impurity phase was detected by FT-IR spectra, and it was in good agreement with the results obtained by powder XRD analysis
3.6 UV–Visible Diffuse Reflectance Spectroscopy Analysis
The optical band gap and the electronic structures of the metal oxide semiconductor materials were determined by UV–Visible absorption/reflectance/transmittance analysis. UV–Visible diffuse reflectance spectroscopy (DRS) studies play a vital role in estimating the energy band gap (E g) of the metal oxide semiconductors. The optical band gap was calculated using Tauc relation [36]. The Kubelka–Munk function is generally applied to convert the DRS into equivalent absorption coefficient and mostly used for analyzing the powder samples [37]. The Kubelka–Munk function F(R) was used to calculate the E g of the spinel NiAl 2 O 4 semiconductors. Thus, the vertical axis is converted into quantity F(R) which is equal to the absorption coefficient. Thus, the α in the Tauc equation is substituted with F(R) and, hence, the relation becomes
where F(R) is the Kubelka–Munk function, α is the absorption coefficient, and R is the reflectance. Thus the Tauc relation becomes
where n = 0.5 and 2 for direct and indirect transitions, respectively, thus giving direct and indirect band gaps. The plots of (F(R)h υ)2 versus h υ for both samples are shown in Fig. 6. Extrapolation of linear regions of these plots to (F(R)h υ)2=0 gives the direct band gap values. The direct band gap (E g) value of the NiAl 2 O 4 NSPs sample was observed to be 3.41 eV, and it is decreased with increasing the higher crystallite size of the NiAl 2 O 4 NPLs sample (3.08 eV). The observed higher E g value of the NiAl 2 O 4 NSPs sample is due to smaller particle size. Interestingly, the energy band gap decreased with increasing the crystallite size of the samples, which obeys the quantum confinement effect
3.7 Photoluminescence Spectroscopy
Room-temperature photoluminescence (PL) spectroscopy gives the information about the band gap with the relative active position of sub-band gap and defect states of the metal oxide semiconductors [38, 39], and it is an important tool for investigating the electronic and optical properties of the semiconducting materials. Figure 7 demonstrates the room-temperature PL spectra recorded at λ ex = 265 nm of NiAl 2 O 4 samples prepared using the two different methods namely MCM and CCM, respectively. A small band observed at 271 nm (4.57 eV) is ascribed to the near-band edge (NBE) emission of the wide band gap of NiAl 2 O 4 due to the recombination of free excitons through an exciton–exciton process. The estimated band gap (3.39 eV) from the NBE emission is in agreement with the band gap estimated from the Kubelka–Munk plot derived from the UV–Visible DRS spectra. However, both the samples show a sharp peak at 389 nm (3.18 eV) which may be ascribed to the oxygen vacancies. Violet emissions centered at 415 nm (2.98 eV) and 444 nm (2.79 eV), due to the radiating defects related to the interface traps existing at the grain boundaries.
Also, blue emissions appeared at 460 nm (2.69 eV) and 482 nm (2.57 eV), which represent a deep level of visible emissions associated with localized levels in the band gap [40, 41]. Green emission centered at 518 nm (2.39 eV) and 572 nm (2.16 eV) may be ascribed to the oxygen vacancies. It is found from the room-temperature PL spectra that the emission characteristics are governed by the defect-controlled processes. The NiAl 2 O 4 NSPs sample shows the higher intensity and may exhibit greater catalytic activity than NiAl 2 O 4 NSPs.
3.8 Vibrating Sample Magnetometer Measurements
Magnetic properties of the samples have been analyzed by room-temperature vibrating sample magnetometer (VSM) analysis. Figure 8 shows the magnetic hysteresis loop of the spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs, prepared using MCM and CCM approaches, respectively. The shape and the area of the M–H hysteresis loops were found to depend strongly on the preparation methods as well as on the ratio of the crystalline phases in the powders. Both the samples displayed the normal (s-shaped) narrow hysteresis loops, and the magnetic parameters like saturation magnetization (M s), remanent magnetization (M r), and coercivity (H c) of the samples are summarized in Table 1. The shape of the M–H hysteresis loop shows a characteristic weak ferromagnetic behavior for both samples. From the VSM measurements, the H c and the M r are estimated to be 64.75 O e and 4.827 × 10 −3 emu/g for the NiAl 2 O 4 NSPs sample, respectively, and 98.25 O e and 5.336 × 10 −3 emu/g for the NiAl 2 O 4 NPLs sample, respectively. The M s of the NiAl 2 O 4 NSPs sample (73.42 × 10 −3 emu/g) is higher than that of the NiAl 2 O 4 NPLs sample (49.16 × 10 −3 emu/g), and both the samples are weak ferromagnetic in nature. The lower H c and M r values confirm that the spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples have soft and weak ferromagnetic nature.
Spinel NiAl 2 O 4 nanocrystals have inverse spinel structure with the ferromagnetic nature, due to the exchange between the ions occupying the tetrahedral (A) and octahedral (B) sites. Also, it has zero net magnetization, because of the complete compensation of the sublattice magnetization [42, 43]. At room temperature, the H c values of the NiAl 2 O 4 NPLs sample is 98.25 O e, which is higher than the NiAl 2 O 4 NSPs sample (64.75 O e), and it is probably attributed to the high shape anisotropy of spinel NiAl 2 O 4 nanocrystals, which allow them in magnetizing in all directions along their easy magnetic axes. As a result, the different M s values of spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples prepared using MCM and CCM, respectively, could be related to the change in the sizes of the particles. It is well known that the magnetic property of nanomaterials is strongly dependent on the shape and sizes of the particles and their crystallinity. However, it is observed that the lower H c and M r values confirm that the NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples have soft and weak ferromagnetic nature [43]
3.9 Catalytic Tests
The catalytic performance of NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples prepared using MCM and CCM, respectively, was evaluated in the oxidation of benzyl alcohol in the presence of 30 % H 2 O 2 as oxidant. In the catalytic oxidation reaction, benzyl alcohols (5 mmol) and H 2 O 2 (5 mmol) as the oxidant were added with 0.5 g of heterogeneous catalyst (NiAl 2 O 4 NSPs or NiAl 2 O 4 NPLs) and the contents were heated at 80 °C in the presence of acetonitrile (5 mmol) as the solvent for 5 h. Catalytic conversion and product selectivity strongly depend on the particle size and surface area of the catalyst which was confirmed by catalytic analysis. The observed catalytic results are summarized in Table 1. The resultant catalysts prepared using the two different methods display clear difference in the selective oxidation of benzyl alcohol into benzaldehyde with 100 % selectivity
3.9.1 Effects of Surface Area
Generally, a high specific surface area has a favorable effect on the activity for heterogeneous catalysis. In order to have an idea of the adsorbance capability of spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs, BET surface area was determined using N 2 adsorption–desorption studies and the observed values are given in Table 1. The surface area of the NiAl 2 O 4 NSPs sample was found to be higher, i.e., 86.77 m 2/g, than that of the NiAl 2 O 4 NPLs sample (69.85 m 2/g), and the observed difference may be due to the smaller particle size of NiAl 2 O 4 NSPs compared with NiAl 2 O 4 NPLs, which is confirmed by HR-SEM and HR-TEM analysis. In this present study, the surface area parameters of the samples varied according to the preparation method. Moreover, it is believed that the high surface area of NiAl 2 O 4 NSPs could enhance the catalytic activity than the lower surface area of the NiAl 2 O 4 NPLs sample. The surface area of NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs samples is 86.77 and 69.85 m 2/g, respectively. It was found that the conversion of benzyl alcohol into benzaldehyde for the NiAl 2 O 4 NSPs sample was higher than that of NiAl 2 O 4 NPLs (Fig. 9). It is mainly due to the uniform distribution with smaller particle size of NiAl 2 O 4 NSPs catalyst. The oxidation of benzyl alcohol into benzaldehyde was achieved with 96.25 % conversion and 100 % selectivity of benzaldehyde using the NiAl 2 O 4 NSPs catalyst, which has higher conversion compared with NiAl 2 O 4 NPLs (86.54 % conversion with 100 % selectivity). The observed higher efficiency of NiAl 2 O 4 NSPs catalyst in this oxidation reaction can be attributed to the high dispersity, and large surface area provides more catalytic active sites for this reaction [44]. High specific surface area of NiAl 2 O 4 NSPs (86.77 m 2/g) was useful to catalytic activity via enhancing the adsorption of benzyl alcohol through the surface of the catalyst and is the determining step in the catalytic reaction [45]. However, the NiAl 2 O 4 NPLs sample shows poor performance, due to the larger particle size with lower surface area (6985 m 2/g). In the MCM approach, the final product is formed within few minutes of time with homogeneous and smaller particle size with higher surface area, which enhances their catalytic activity compared with NiAl 2 O 4 NPLs. In addition, when the surface area increases, the amount of the dispersion of particles per volume in the solution will increase and this enhanced the catalytic activity.
3.9.2 Effect of Reaction Time
Catalytic reaction time is very important to measure the activity of catalyst. Figure 9 shows the effect of reaction time on the conversion of benzyl alcohol into benzaldehyde using a heterogeneous (NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs) catalyst. It was found that the conversion increased linearly by increasing the reaction time up to 180 min and then increasing it slowly from 180 to 240 min. The product conversion was found to be nearly the same for the 240- and 300-min experiments, indicating the constant conversion after 240 min. No significant change in the oxidation of benzyl alcohol into benzaldehyde was observed when running the reaction for 300 min [46]. However, the catalytic activity of the NiAl 2 O 4 NSPs sample is higher than that of the NiAl 2 O 4 NPLs sample, due to the smaller particle size distribution which would be a potentially efficient catalyst [47].
3.9.3 Effect of Catalyst Amount
The influence of the catalyst amount on the conversion towards the oxidation of benzyl alcohol into benzaldehyde is shown in Fig. 10. The conversion is gradually increased with increase in the amount of catalyst from 0.1 to 0.5 g, (reaction conditions: catalyst, 0.1 to 0.8 g; benzyl alcohol, 5 mmol; acetonitrile, 5 mmol; H 2 O 2, 5 mmol; temperature, 80 ∘C; time, 5 h), and further increasing the catalyst amount (0.6 to 0.8 g), the conversion and yield remain nearly the same, which suggests that a large amount of catalyst is not needed to improve the reaction product [46]. However, it is found that with the increase of catalyst amount, the conversion and yield increased up to 0.5 g and this may be apparently due to the availability of more catalytic active sites of the catalyst.
3.9.4 Reusability Studies
The catalytic oxidation of benzyl alcohol into benzaldehyde was employed as a model reaction to investigate the reusability of spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs catalysts. The recycling of the catalyst is very important for industrial and technological applications for reusability of the catalysts. The reusability of the catalyst for the liquid-phase oxidation of benzyl alcohol into benzaldehyde was evaluated, and the results are shown in Fig. 11. For the reusability study purpose, the spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs sample catalysts were filtered off from each run, washed several times with ethanol, dried at 110 ∘C in an air oven for 1 h, and checked for five consecutive runs under the identical conditions. During the five runs investigated, the conversion of benzyl alcohol into benzaldehyde was in the range from 96.25 to 95.13 % for the NiAl 2 O 4 NSPs sample (Fig. 11a), indicating that this catalyst displays good reproducibility and stability. Interestingly, the formation of benzoic acid was not detected. However, it was observed that the conversion of benzyl alcohol into benzaldehyde was in the range from 86.84 to 84.15 % for the NiAl 2 O 4 NPLs sample (Fig. 11b). Since the spinel NiAl 2 O 4 nanocatalyst is able to oxidize benzyl alcohol into benzaldehyde with high activity, highly recyclable, remarkably stable, and environmental friendly, they are promising candidates for the industrial and technological applications [48, 49].
3.9.5 Proposed Catalytic Oxidation Reaction Mechanism
In the proposed catalytic oxidation reaction mechanism for NiAl 2 O 4 catalyst, the solvent CH 3CN can activate oxidant H 2 O 2 by forming a perhydroxyl anion, OOH −, to generate an intermediate peroxycarboximidic acid. It is well known that the formed intermediate peroxycarboximidic acid is a good oxygen transfer agent. The NiAl 2 O 4 catalyst reacts with the H 2 O 2 molecule to form Ni–OOH − as an initiation step in this reaction. This perhydroxyl species, which are formed on the surface of the catalyst, will then oxidize the alcohol substrates. The benzyl alcohol and the H 2 O 2 dissolve in CH 3CN, which activate H 2 O 2 to OOH − anion, forming Ni–OOH − species [50]. This account revealed that the OH group of benzyl alcohols interacts with the Ni 2+ ion. The contact of the OH group with the Ni 2+ ions in NiAl 2 O 4 is also clear by the adsorption of phenyl ring on the catalyst. However, the NiAl 2 O 4 catalyst surface gets regenerated by the action of H 2 O 2, which leads to desorption of the carbonyl product (benzaldehyde), thus favoring further oxidation. In addition, H 2 O 2 has been proved to be a very efficient and environmentally friendly oxidant, since the product is only carbonyls [46, 47]. Usually, the catalytic oxidation of benzyl alcohol produces a mixture of carbonyls and carboxylic acids. However, carboxylic acid is not obtained in the present study. Therefore, the heterogeneous catalyst was found to be highly selective [51].
4 Conclusions
Spinel NiAl 2 O 4 NSPs and NiAl 2 O 4 NPLs have been successfully synthesized using MCM and CCM techniques, respectively, using urea as the fuel without any other solvent, surfactant, and catalyst. MCM approach is extremely facile and offers an inexpensive method for the preparation of nanostructured functional materials than CCM route. Powder XRD, FT-IR, EDX spectra, SAED, HR-SEM, and HR-TEM results showed that the as-prepared samples were pure phase spinel NiAl 2 O 4 nanosphere and nanoplatelet-like particle morphologies with good crystalline nature prepared using MCM and CCM, respectively, without any other secondary phase impurity. The calculated optical band gap value of NiAl 2 O 4 NSPs is 3.41 eV, which is higher than that of the NiAl 2 O 4 NPLs sample (3.08 eV), due to the smaller particle size. The relatively lower M s (49.16 × 10 −3 emu/g) of NiAl 2 O 4 NPLs shows that it is weak ferromagnetic in nature, and the high M s (73.42 × 10 −3 emu/g) of NiAl 2 O 4 NSPs confirms its ferromagnetic behavior. It was found that the NiAl 2 O 4 NSPs sample shows higher conversion (96.25 %) than NiAl 2 O 4 NPLs (84.15 %). Moreover, the MCM approach provides a simple and convenient method for preparing the transition spinel metal oxides. The synthetic route can be further used for the synthesis of other nanometal oxides.
References
Baykal, A., Deligoz, H., Sozeri, H., Durmus, Z., Toprak, M. S.: J. Supercond. Nov. Magn 25, 1879 (2012)
Karaoglu, E., Baykal, A.: J Supercond. Nov. Magn 27, 2041 (2014)
Amir, Md., Sertkol, M., Baykal, A., Sozeri, H.: J Supercond. Nov. Magn 28, 2447 (2015)
Genc, F., Unal, B., Baykal, A., Sozeri, H.: J Supercond. Nov. Magn 28, 1055 (2015)
Amir, Md., Baykal, A., Guner, S., Sertkol, M., Sozeri, H., Toprak, M.: J Inorg. Organomet. Polym 25, 747 (2015)
Amir, Md., Baykal, A., Sertkol, M., Sozeri, H.: J Inorg. Organomet. Polym 25, 619 (2015)
Guner, S., Amir, Md., Geleri, M., Sertkol, M., Baykal, A.: Ceram. Int 41, 10915 (2015)
Amir, Md., Unal, B., Shirsath, S.E., Geleri, M., Sertkol, M., Baykal, A.: Superlatt. Microstruct 85, 747 (2015)
Gozüak, F., Koseoglu, Y., Baykal, A., Kavas, H.: J. Magn. Magn. Mater 321, 2170 (2009)
Koseoglu, Y., Baykal, A., Gozuak, F., Kavas, H.: Polyhedron 28, 2887 (2009)
Manikandan, A., Durka, M., Arul Antony, S.: J Supercond. Nov. Magn 28, 209 (2015)
Manikandan, A., Durka, M., Arul Antony, S.: J Supercond. Nov. Magn 27, 2841 (2014)
Manikandan, A., Sridhar, R., Arul Antony, S., Ramakrishna, S.: J. Mol. Struct 1076, 188 (2014)
Manikandan, A., Durka, M., Seevakan, K., Arul Antony, S.: J Supercond. Nov. Magn 28, 1405 (2015)
Manikandan, A., Arul Antony, S., Sridhar, R., Bououdina, M.: J. Nanosci. Nanotechnol 15, 4948 (2015)
Manikandan, A., Durka, M., Arul Antony, S.: Adv. Sci. Eng. Med 7, 33 (2015)
Manikandan, A., Hema, E., Durka, M., Seevakan, K., Alagesan, T., Arul Antony, S.: J Supercond. Nov. Magn 28, 1783 (2015)
Han, Y.S., Li, J.B., Ning, X.S., Chi, B.: J. Am. Ceram. Soc 88, 3455 (2005)
Fonseca, R.L., Gonzalez, C.J., Rivas, B., Ortiz, J.I.G.: Appl. Catal. A 437, 53 (2012)
Li, G., Hu, L., Hill, J.M.: Appl. Catal. A 301, 16 (2006)
Cesteros, Y., Salagre, P., Medina, F., Sueiras, J.E. Chem. Mater 12, 331 (2000)
Cui, H., Zayat, M., Levy, D.: J. Non-Cryst. Solids 351, 2102 (2005)
Seo, J., Youn, M., Cho, K., Park, S., Lee, S., Lee, J., Song, I.: Korean J. Chem. Eng 25, 41 (2008)
Hosseini, S.A., Niaei, A., Salari, D., Nabavi, S.R.: Ceram. Int 38, 1655 (2012)
Valan, M.F., Manikandan, A., Arul Antony, S.: J. Nanosci. Nanotechnol 15, 4580 (2015)
Valan, M.F., Manikandan, A., Arul Antony, S.: J. Nanosci. Nanotechnol 15, 4543 (2015)
Manikandan, A., Arul Antony, S.: J Supercond. Nov. Magn 27, 2725 (2014)
Manikandan, A., Durka, M., Arul Antony, S. J Inorg. Organomet. Polym 25, 804 (2015)
Manikandan, A., Durka, M., Arul Antony, S.: J. Inorg. Organomet. Polym. 25, 1019 (2015)
Manikandan, A., Durka, M., Arul Antony, S.: J Supercond. Nov. Magn 28, 2047 (2015)
Meyer, F., Hempelmann, R., Mathurband, S., Veith, M.: J. Mater. Chem 9, 1755 (1999)
Manikandan, A., Saravanan, A., Arul Antony, S., Bououdina, M.: J. Nanosci. Nanotechnol 15, 4358 (2015)
Chinnaraj, K., Manikandan, A., Ramu, P., Arul Antony, S., Neeraja, P.: J Supercond. Nov. Magn 28, 179 (2015)
Jeevanandam, P., Koltypin, Y., Gedanken, A.: Mater Sci. Eng. B 90, 125 (2002)
Bayal, N., Jeevanandam, P.: J. Alloys Compd 516, 27 (2012)
Wang, S.F., Gu, F., Lu, M.K., Cheng, X.F., Zou, W.G., Zhou, G.J., Wang, S.M., Zhou, Y.Y.: J. Alloys Compd 394, 255 (2005)
Bai, H., Liu, Z., Sun, D.D.: Int. J. Hydrogen Energy 37, 13998 (2012)
Schmidt, T., Lischka, K., Zulehner, W.: Phys. Rev. B 45, 8989 (1992)
Sendi, R.K., Mahmudm, S.: Appl. Surf. Sci 258, 8026 (2012)
Bhargava, R., Sharma, P.K., Dutta, R.K., Kumar, S., Pandey, A.C., Kumar, N.: Mater. Chem. Phys 120, 393 (2010)
Shen, Y., Li, W., Li, T.: Mater. Lett 65, 2956 (2011)
Ichiyanagi, Y., Kimishima, Y., Yamada, S.: J. Magn. Magn. Mater 272, E1245 (2004)
Bhatt, A.S., Bhat, D.K., Tai, C., Santosh, M.S.: Mater. Chem. Phys 125, 347 (2011)
Albuquerque, A.S., Tolentino, M.V., Ardisson, J.D., Moura, F.C., Mendonca, R., Macedo, W.A.: Ceram. Int 38, 2225 (2012)
Hankare, P.P., Kamble, P.D., Maradur, S.P., Kadam, M. R., Sankpal, U.B., Patil, R.P., Nimat, R.K., Lokhande, P.D.: J. Alloys Compd 487, 730 (2009)
Ali, S.R., Chandra, P., Latwal, M., Jain, S.K., Bansal, V.K., Singh, S.P.: Chin. J. Catal 32, 1844 (2011)
Ma, C.Y., Cheng, J., Wang, H.L., Hu, Q., Tian, H., He, C., Hao, Z.P.: Catal. Today 158, 246 (2010)
Seo, J.G., Youn, M.H., Park, S., Park, D.R., Jung, J.C., Chung, J.S., Song, I.K.: Catal. Today 146, 44 (2009)
Loginova, E., Cosandey, F., Madey, T. E.: Surf. Sci 601, L11 (2007)
Boudreau, M.D., Beland, F.A.: J Environ. Sci. Health Part C 24, 103 (2006)
Hema, E., Manikandan, A., Karthiga, P., Durka, M., Arul Antony, S., Venkatraman, B.R.: J Supercond. Nov. Magn 28, 253 (2015)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jayasree, S., Manikandan, A., Antony, S.A. et al. Magneto-Optical and Catalytic Properties of Recyclable Spinel NiAl 2 O 4 Nanostructures Using Facile Combustion Methods. J Supercond Nov Magn 29, 253–263 (2016). https://doi.org/10.1007/s10948-015-3249-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-015-3249-5