Abstract
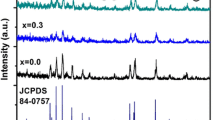
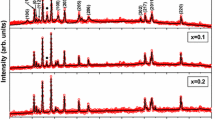
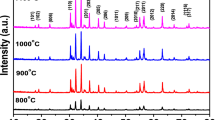
The barium and strontium hexaferrites (BaM and SrM) were successfully prepared by the standard solid-state reaction method. To inhibit crystal growth, 1 wt % B2O3 was added into the initial mixture. The structural and magnetic properties of pure hexaferrites were compared with the samples prepared with boron addition. Powders were annealed at temperatures between 800 and 1200 ∘C. The crystal structure, morphology, and magnetic properties of hexaferrites were investigated with X-ray diffraction (XRD), scanning electron microscopy (SEM), and vibrating sample magnetometer (VSM). BaM was synthesized at temperatures as low as 800 ∘C with boron addition having saturation magnetization of ∼50 emu/g and coercivity of ∼3 kOe. At the same temperature, pure sample has very low magnetization about 1.3 emu/g. Moreover, at higher sintering temperatures, boron-containing samples have higher magnetization values compared to pure BaM. The optimal Fe/Ba ratio was determined as 10.5 for boron-containing samples, while it is 11.5 for the pure one. The coercivity started to decrease at 1100 ∘C indicating that single to multi-domain transition occurs. The saturation magnetization values are nearly equal in pure- and boron-containing SrM samples in the whole temperature range. However, maximal coercivities occurred at different temperatures for boron-containing and the pure samples as 1000 and 1100 ∘C, respectively. Magnetic interactions deduced from the Stoner–Wohlfarth model using the isothermal magnetization and demagnetization measurements showed that boron addition suppresses the destructive (demagnetizing-like) interactions among domains in both SrM and BaM samples, and thus, stabilizes the remanence.








Similar content being viewed by others
References
Xie, T., Xu, L., Liu, C.: Synthesis and properties of composite magnetic material SrCoxFe12-xO19 (x  = 0–0.3). Powder Technol. 232(0), 87–92 (2012)
Jean, M., et al.: Synthesis and characterization of SrFe12O19 powder obtained by hydrothermal process. J. Alloys Compd. 496(1–2), 306–312 (2010)
Sözeri, H., Baykal, A., Ünal, B.: Low-temperature synthesis of single-domain Sr-hexaferrite particles by solid-state reaction route. Phys. Status Solidi (a) 209(10), 2002–2013 (2012)
Pramanik, N.C., et al.: Development of nanograined hexagonal barium ferrite thin films by sol–gel technique. Mater. Lett. 59(4), 468–472 (2005)
Le Breton, J.-M., et al.: Structural analysis of co-precipitated Sr1−x La x Fe12−x Co x O19 powders. J. Magn. Magn. Mater. 272–276, Part 3(0), 2214–2215 (2004)
Zaitsev, D.D., et al.: Preparation of the SrFe12O19-based magnetic composites via boron oxide glass devitrification. J. Magn. Magn. Mater. 301(2), 489–494 (2006)
Bish, D.L., Howard, S.A.: Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 21(2), 86–91 (1988)
Thompson, G.K., Evans, B.J.: The structure–property relationships in M-type hexaferrites: Hyperfine interactions and bulk magnetic properties. J. Appl. Phys. 73(10), 6295–6297 (1993)
Mariño-Castellanos, P.A., Somarriba-Jarque, J.C., Anglada-Rivera, J.: Magnetic and microstructural properties of the BaFe(12−(4/3)x)Sn x O19 ceramic system. Phys. B Condens. Matter 362(1–4), 95–102 (2005)
Wejrzanowski, T., et al.: Quantitative methods for nanopowders characterization. Appl. Surf. Sci. 253 (1), 204–208 (2006)
Solanki, N., Packiaraj, G., Jotania, R.B.: Effect of heat treatment on structural, magnetic and electric properties of Z-type barium cobalt hexaferrite powder. In: Advanced Materials Research 2014, pp. 24–29
Zhong, W., et al.: Key step in synthesis of ultrafine BaFe12O19 by sol–gel technique. J. Magn. Magn. Mater. 168(1–2), 196–202 (1997)
Li, Y., Wang, Q., Yang, H.: Synthesis, characterization and magnetic properties on nanocrystalline BaFe12O19 ferrite. Curr. Appl. Phys. 9(6), 1375–1380 (2009)
Xu, G., et al.: Influence of pH on characteristics of BaFe12O19 powder prepared by sol–gel auto-combustion. J. Magn. Magn. Mater. 301(2), 383–388 (2006)
Mali, A., Ataie, A.: Influence of Fe/Ba molar ratio on the characteristics of Ba-hexaferrite particles prepared by sol–gel combustion method. J. Alloys Compd. 399(1–2), 245–250 (2005)
Janasi, S.R., et al.: Magnetic properties of coprecipitated barium ferrite powders as a function of synthesis conditions. Magn. IEEE Trans. 36(5), 3327–3329 (2000)
Liu, X., et al.: Improving the magnetic properties of hydrothermally synthesized barium ferrite. J. Magn. Magn. Mater. 195(2), 452–459 (1999)
Pullar, R.C., Bhattacharya, A.K.: Crystallisation of hexagonal M ferrites from a stoichiometric sol–gel precursor, without formation of the α-BaFe2O4 intermediate phase. Mater. Lett. 57(3), 537–542 (2002)
Sözeri, H., et al.: Preparation of high quality, single domain BaFe12O19 particles by the citrate sol–gel combustion route with an initial Fe/Ba molar ratio of 4. Mater. Sci. Eng. B 177(12), 949–955 (2012)
Sözeri, H., Küçük, İ., Özkan, H.: Improvement in magnetic properties of La substituted BaFe12O19 particles prepared with an unusually low Fe/Ba molar ratio. J. Magn. Magn. Mater. 323(13), 1799–1804 (2011)
Liu, W.-T., Wu, J.-M.: The effect of the vacuum extraction and the Fe/Ba ratio on the phase formation of barium ferrite thin film synthesized by sol–gel method. Mater. Chem. Phys. 69(1–3), 148–153 (2001)
Sürig, C., Hempel, K.A., Sauer, C.: Influence of stoichiometry on hexaferrite structure. J. Magn. Magn. Mater. 157–158, 268–269 (1996)
Wohlfarth, E.P.: Relations between different modes of acquisition of the remanent magnetization of ferromagnetic particles. J. Appl. Phys. 29(3), 595–596 (1958)
Acknowledgments
This work is supported by TUBITAK (the Scientific and Technological Research Council of Turkey) with Project Number 213M174.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehmedi, Z., Sözeri, H., Topal, U. et al. Effect of Annealing Temperature and Boron Addition on Magnetic Properties of Hexaferrites Synthesized by Standard Ceramic Method. J Supercond Nov Magn 28, 1395–1404 (2015). https://doi.org/10.1007/s10948-014-2865-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2865-9