Abstract
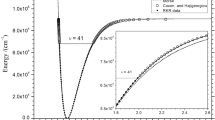
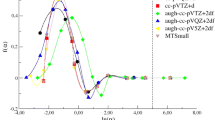
The vaporization enthalpies of mono- and dicarbonic acids with different structures are calculated under normal conditions using the modified Randič method and the energies of hydrogen bonds. The results demonstrate good agreement between the experimental and calculated data.
Similar content being viewed by others
References
E. L. Krasnykh, J. Struct. Chem., 49, No. 6, 986–993 (2008).
J. Konicek and I. Wadso, Acta Chem. Scand., 24, 2612 (1970).
S. P. Verevkin, J. Chem. Eng. Data, 45, 953 (2000).
C. G. Kruif and H. A. J. Oonk, J. Chem. Thermodyn., No. 11, 287 (1979).
D. Ambrose and N. B. Ghiassee, ibid., 19, 505 (1987).
C. D. Cappa, E. R. Lovejoy, and A. R. Ravishankara, J. Phys. Chem. A, 112, 3959 (2008).
S. P, Verevkin E. L. Krasnykh, T. V. Vasiltsova, and A. Heintz, J. Chem. Eng. Data, 48, 591 (2003).
S. V. Lipp, E. L. Krasnykh, and S. V. Levanova, Zh. Fiz. Khim., 82, No. 12, 2250 (2008).
J. Marrero and R. Gani, Fluid Phase Equil., 183 (2001).
M. Ducros, J. F. Gruson, and H. Sannier, Thermochim. Acta, 36, 39 (1980).
P. E. Hintze, S. Aloisio, and V. Vaida, Chem. Phys. Lett., 343, 159 (2001).
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem., 14, 1347 (1993).
P. Gilli, L. Pretto, V. Bertolasi, and G. Gilli, Acc. Chem. Res., 42, No. 1, 33 (2009).
A. C. Vawdrey, J. L. Oscarson, R. L. Rowley, and W. V. Wilding, Fluid Phase Equil., 222 (2004).
E. L. Krasnykh, J. Struct. Chem., 50, No. 3, 577 (2009).
L. A. McDougall and J. E. Kilpatrick, J. Chem. Phys., 42, 2307 (1965).
W. V. Steele, R. D. Chirico, A. B. Cowell, et al., J. Chem. Eng. Data, 47(4), 700 (2002).
W. V. Steele, R. D. Chirico, S. E. Knipmeyer, and A. Nguyen, J. Chem. Eng. Data, 42, 1021 (1997).
W. V. Steele, R. D. Chirico, S. E. Knipmeyer, and A. Nguyen, ibid., 47, 648 (2002).
M. J. Wojcik, J. Mol. Struct., 225 (2005).
M. V. Roux, M. Temprado, and J. S. Chickos, J. Chem. Thermodyn., 37, 941 (2005).
I. Riipinen, I. K. Koponen, G. P. Frank, et al., J. Phys. Chem. A, 111, 12995 (2007).
M. Bilde, B. Svenningsson, J. Mnster, and T. Rosenrn, Environ. Sci. Technol., 37, 1371 (2003).
R. Saleha, J. Walkerb, and A. Khlystova, Aerosol Science, 39, 876 (2008).
W. V. Steele, R. D. Chirico, A. B. Cowell, et al., J. Chem. Eng. Data, 47, 725 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2010 by E. L. Krasnykh
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 51, No. 2, pp. 231–236, March–April, 2010.
Rights and permissions
About this article
Cite this article
Krasnykh, E.L. Prediction of the vaporization enthalpy based on modified randič indices. III. Carbonic acids. J Struct Chem 51, 217–222 (2010). https://doi.org/10.1007/s10947-010-0034-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10947-010-0034-y