Abstract
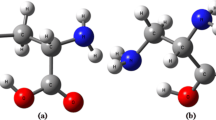
A theoretical study of the interaction between the N-nitrosodiethanolamine (NDELA) molecule and one to five water molecules was performed at the B3LYP level using a large polarized basis set. The calculated complexation energies (corrected for BSSE and ZPVE) of NDELA with one, two, three, four, and five water molecules are −4.62 kcal/mol, −9.83 kcal/mol, −15.29 kcal/mol, −21.60 kcal/mol, and −25.10 kcal/mol respectively at the B3LYP/6-311++G** level. In all complexes studied, there are red shifts in the vibrational frequencies of the O-Hs of NDELA and water molecules along with increases in the corresponding IR intensities.
Similar content being viewed by others
References
J. You, X. Fan, W. Lao, et al., Talanta, 48, 437 (1999).
W. Zwickenpflug and E. Ritcher, J. Chromatogr. Sci., 25, 506 (1987).
C. P. Oliveria, M. B. A. Glória, J. F. Barbour, and R. A. Scanlan, J. Agric. Food Chem., 43, 967 (1995).
N. P. Sen, S. W. Seaman, P. A. Baddoo, and D. Weber, J. Food Sci., 53, 731 (1988).
R. J. Maxwell, J. W. Pensabene, and W. Fiddler, J. Chromatogr. Sci., 31, 212 (1993).
B. A. Tomkins and W. H. Griest, Anal. Chem., 68, 2533 (1996).
H. D. Fine, R. Ross, D. P. Rounbehler, et al., J. Agric. Food Chem., 24, 1069 (1976).
N. P. Sen, S. W. Seaman, and S. C. Kushwaha, J. Chromatogr., 463, 419 (1989).
M. B. A. Glória, J. F. Barbour, and R. A. Scanlan, J. Agric. Food Chem., 45, 814 (1997).
R. W. Stephany, J. Freudenthal, E. Egmond, et al., ibid., 24, 536 (1976).
B. Spiegelhalder and R. Preussmann, J. Cancer Res. Clin. Oncol., 108, 160 (1984).
V. Barone, P. Palma, and N. Sanna, Chem. Phys. Lett., 381, 451 (2003).
Q. S. Li and W. H. Fang, ibid., 367, 637.
D. Wang, S. Chen, and D. Chen, J. Photochem. Photobiol. A., 162, 407 (2004).
S. Scheiner, Hydrogen Bonding, A Theoretical Perspective, Oxford University Press, Oxford (1997).
L. Pejov, M. Solimannejad, and V. Stefov, Chem. Phys., 323, 259 (2006).
M. Solimannejad and S. Scheiner, Chem. Phys. Lett., 429, 38 (2006).
M. Solimannejad and S. Scheiner, ibid., 424, 1.
M. Solimannejad and S. Scheiner, J. Phys. Chem., A110, 5948 (2006).
M. Solimannejad and I. Alkorta, ibid, 10817.
M. Solimannejad and M. E. Alikhani, Chem. Phys. Lett., 406, 351 (2005).
M. Solimannejad, G. Azimi, and L. Pejov, ibid., 400, 185 (2004).
M. Solimannejad and L. Pejov, ibid., 385, 394 (2004).
M. J. Frisch et al., Gaussian-03, Revision B02, Gaussian, Inc., Pittsburgh (2003).
S. F. Boys and F. Bernardi, Mol. Phys., 19, 553 (1970).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2008 by R. Alizadeh and N. M. Najafi
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 49, No. 4, pp. 649–654, July–August, 2008.
Rights and permissions
About this article
Cite this article
Alizadeh, R., Najafi, N.M. Theoretical study of the structure, stability, and infrared spectra of hydrogen-bonded complexes of N-nitrosodiethanolamine (NDELA) with water molecules. J Struct Chem 49, 621–626 (2008). https://doi.org/10.1007/s10947-008-0086-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10947-008-0086-4