Abstract
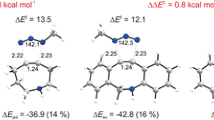
Using RHF/3-21G, RHF/6-31G(d, p), MP2/6-31G(d, p), B3LYP/6-31G(fd, p) approximations the structure and 13C NMR spectra of 2-alkylsubstituted cyclohexene-2-ones and 2-alkylacroleins are studied and calculated. In the series of 2-alkylcyclohexene-2-ones the effect of the substituent on a deviation from coplanarity of the C=C-C=O fragment is more expressed in comparison with 2-alkylacroleins. This deviation (5°) is not enough to explain the observed properties of 2-alkylcyclohexene-2-ones due to disturbed conjugation. The particular behavior of (R)-4-mentenone in reactions of 1,4-addition and ozonolysis is explained by a more expressed +I-effect of the alkyl substituent in α-position.
Similar content being viewed by others
References
G. M. Whitesidea, W. F. Fischer, J. S. Fillipo, et al., J. Am. Chem. Soc., 91, No. 17, 4871–4882 (1969).
G. H. Posner, Org. React., 19, 293–296 (1972).
É. R. Latypova, R. Ya. Kharisov, G. Yu. Ishmuratov, and R. F. Talipov, (R)-4-Mentenone in the Reaction of Conjugated 1,4-Addition of Organometallic Reagents, Proceedings of the 4th International Conference of Young Scientists “Current tendencies in organic synthesis and problems of chemical education” [in Russian], St.Petersburg (2005).
R. Ya. Kharisov, R. R. Gazetdinov, O. V. Botsman, et al., Zh. Org. Khim., 36, No. 7, 1047–1050 (2002).
M. W. Smidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem., 14, 1347–1363 (1993).
F. Jensen, Introduction to Computational Chemistry, Wiley, Chichester (1999).
J. A. Pople, P. M. W. Gill, and B. G. Johnson, Chem. Phys. Lett., No. 199, 557–570 (1992).
W. Kohn, A. D. Backe, and R. G. Parr, J. Phys. Chem., 100, 12974–12980 (1996).
W. J. Hehre, L. Radom, P. V. R. Schleyer, et al., Ab initio Molecular Orbital Theory, Wiley-Interscience, New York (1985).
C. Kubli-Garfias, J. Mol. Struct. (Thechem), 422, 167–177 (1998).
C. Kubli-Garfias and R. Vazguez-Ramirez, ibid., 454, 267–275.
General Organic Chemistry, 2 [in Russian], Khimiya, Moscow (1982).
J. March, Advanced Organic Chemistry, McGraw Hill (1968).
J. P. Collman, L. S. Hegedus, J. R. Norton, and R. G. Finke Principles and Applications of Organotransition Metal Chemistry, University Science Books: Mill Valley, California (1987).
B. A. Ershov, B. I. Ionin, et al., NMR Spectroscopy in Organic Chemistry [in Russian], Izd-vo Leningr. Univ., Leningrad (1983).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2007 by É. R. Latypova, R. Ya. Kharisov, I. V. Vakulin, R. F. Talipov, and G. Yu. Ishmuratov
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 48, No. 1, pp. 49–53, January–February, 2007.
Rights and permissions
About this article
Cite this article
Latypova, É.R., Kharisov, R.Y., Vakulin, I.V. et al. Electronic effects of conjugated enones on their reactivity in transformations of ADDN type. J Struct Chem 48, 46–50 (2007). https://doi.org/10.1007/s10947-007-0007-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-007-0007-y