Abstract
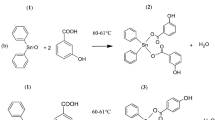
The reaction of VO(acac)2 (acac = acetylacetonate) with NaHB(pz)3 (pz = pyrazole) or NaHB(3,5-Me2pz)3 in methanol gave vanadium(IV) complexes HB(pz)3VO(acac) (1) or HB(3,5-Me2pz)3VO(acac)·CH3CN (2), respectively. The complexes 1 and 2 were characterized by elemental analysis, IR, UV-vis, NMR and X-ray diffraction crystallography methods. Complex 1 crystallizes in space group P21/c, a = 7.641(2) Å, b = 17.008(4) Å, c = 13.362(2) Å; β = 92.092(17)°, V = 1735.5(7) Å3, Z = 4. Complex 2 crystallizes in space group P21/c, a = 17.410(13) Å, b = 8.076(16) Å, c = 19.300(13) Å; β = 101.75(5)°, V = 2657(6) Å3, Z = 4. X-ray structure analyses have shown that the complexes 1 and 2 are monomeric with a similar coordination environment of the vanadium atom. Luminescence properties and cytotoxic effects of the complexes are discussed. On CBRH-7919 cells, the complexes 1 and 2 caused a slight stimulation of growth at low doses (1–10 µM) and a significant cytotoxic effect at higher doses (100–1000 µM). The electronic structure and the bonding characters of the two complexes were analyzed with ab initio calculations.
Similar content being viewed by others
References
R. R. Eady, Coord. Chem. Rev., 237, 23 (2003).
M. Ahmed, P. Schwendt, J. Marek, and M. Sivak, Polyhedron, 23, 655 (2004).
I. Osinska-Krolicka, H. Podsiadly, A. Bukietynska, et al., J. Inorg. Biochem., 98, 2087 (2004).
J. McMaster, Annu. Rep. Prog. Chem., Sect, A98, 593 (2002).
J. Blower, ibid., 615.
H. Sakurai, A. Tamura, T. Takino, et al., Inorg. React. Mechan., 2, 69 (2000).
Y. Shechter and S. J. D. Karlish, Nature, 286, 556 (1980).
C. E. Heyliger, A. G. Tahiliani, and J. H. McNeil, Science, 227, 1474 (1985).
G. R. Dubyak and A. Kleinzeller, J. Biol. Chem., 255, 5306 (1980).
J. Meyerovitch, Z. Farfel, J. Sack, and Y. Shechter, ibid., 262, 6658 (1987)
Y. Shechter, Diabetes, 39, 1 (1990).
A. J. Stemmler and C. J. Burrows, J. Biol. Inorg. Chem., 6, 100 (2001).
K. H. Thompson and C. Orvig, J. Chem. Soc., Dalton Trans., 2885 (2000).
D. Rehder, J. C. Pessoa, C. F. G. Geraldes, et al., J. Biol. Inorg. Chem., 7, 384 (2002).
H. Sakurai, K. Tsuchiya, M. Nukatsuka, et al., J. Clin. Biochem. Nutr., 8, 193 (1990).
Y. Shechter, A. Shisheva, R. Lazar, et al., Biochem., 31, 2063 (1992).
D. C. Crans, J. J. Smee, E. Gaidamauskas, and L. Q. Yang, Chem. Rev., 104, 849 (2004).
G. Micera, D. Sanna, E. Kiss, et al., J. Inorg. Biochem., 75, 303 (1999).
L. L. G. Justino, M. L. Ramos, M. M. Caldeira, and V. M. S. Gil, Inorg. Chim. Acta, 356, 179 (2003).
W. Plass, Coord. Chem. Rev., 237, 205 (2003).
N. Kitajima and W. B. Tolman, Progr. Inorg. Chem., 43, 419 (1995).
S. Trofimenko, Chem. Rev., 72, 497 (1972).
B. Machura, J. O. Dziegielewski, R. Kruszynski, et al., Inorg. Chim. Acta, 357, 1011 (2004).
E. Gutierrez, S. A. Hudson, A. Monge, et al., J. Organomet. Chem., 551, 215 (1998).
H. V. R. Dias and X. Y. Wang, Polyhedron, 23, 2533 (2004).
N. S. Dean, M. R. Bond, C. J. O’Connor, and C. J. Carrano, Inorg. Chem., 35, 7643 (1996).
S. Holmes and C. J. Carrano, ibid., 30, 1231 (1991).
E. Kime-Hunt, K. Spartalian, M. DeRusha, et al., ibid., 28, 4392 (1989).
J. Reglinski, M. Garner, I. D. Cassidy, et al., J. Chem. Soc., Dalton Trans., 2119 (1999).
S. Trofimenko, J. Am. Chem. Soc., 89, 6288 (1967).
G. M. Sheldrick, Acta Crystallogr., A46, 467 (1990).
R. Herbst-Irmer and G. M. Sheldrick, ibid., B54, 443 (1998).
L. J. Farrugia, J. Appl. Crystallogr., 30, 565 (1997).
R. Garcia, Y. H. Xing, A. Domingos, et al., Inorg. Chim. Acta, 343, 27 (2003).
R. L. Beddoes, D. Collison, F. E. Mabbs, and M. A. Passand, Polyhedron, 9, 2483 (1990).
S. S. Amin, K. Cryer, B. Y. Zhang, et al., Inorg. Chem., 39, 406 (2000).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2006 by Y. H. Xing, Z. Sun, W. Zou, J. Song, K. Aoki, and M. F. Ge
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 47, No. 5, pp. 924–932, September–October, 2006.
Rights and permissions
About this article
Cite this article
Xing, Y.H., Sun, Z., Zou, W. et al. Synthesis, structure, luminescence properties, quantum chemistry and cytotoxic effects of two vanadium(IV) complexes with polypyrazolylborates, HB(pz)3VO(acac) and HB(3,5-Me2pz)3VO(acac)·CH3CN (pz = pyrazole). J Struct Chem 47, 913–922 (2006). https://doi.org/10.1007/s10947-006-0408-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0408-3