Abstract
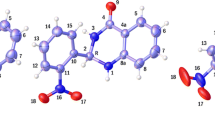
A non-empirical quantum chemical calculation of isomeric 3,6-divinyl-3,4,5,6-tetrahydropyrrolo[3,2-e] indole 1 and 1,5-divinyl-1,4,5,8-tetrahydro[3,2-f]indole 2 structures carried out by DFT (B3LYP) method with 6-311++G(d, p) and 6-311++G(3df, p) basis sets showed the energy preference of 2 over 1 (1.33 kcal/mol and 1.47 kcal/mol, respectively). The structure of the molecule of 2 is planar while the molecule of 1 is non-planar due to the presence of sp 3-hybridized carbon atoms.
Similar content being viewed by others
References
F. Lucchesini, Tetrahedron, 48, 9951–9966 (1992).
J. Roncali, Chem. Rev., 92, 711–732 (1992).
A. R. Katritzky, J. Li, and M. F. Gordeev, Synthesis, 93–96 (1994).
T. E. O. Screen, K. B. Lawton, G. S. Wilson, et al., J. Mater. Chem., 11, 312–320 (2001).
A. O. Patil, A. J. Heeger, and F. Wudl, Chem. Rev., 88, 183–200 (1988).
J. Eldo, E. Arunkumar, and A. Ajayaghosh, Tetrahedron Lett., 41, 6241–6244 (2000).
M. Omastova, M. Trchova, J. Kovarova, and J. Stejskal, Synth. Met., 138, 447–455 (2003).
D. Aldakov and P. Jr. Anzenbacher, Chem. Commun., 1394/1395 (2003).
J. Sołoducho, J. Doskocz, J. Cabaj, and S. Roszak, Tetrahedron., 59, 4761–4766 (2003).
H. Z. Chen, Y. D. Jin, R. S. Xu, et al., Synth. Met., 139, 529–534 (2003).
J. J. Klappa, S. A. Geers, S. J. Smidtke, et al., Dalton Trans., 883–891 (2004).
B. A. Trofimov, A. B. Zaitsev, E. Yu. Schmidt, et al., Tetrahedron Lett., 45, 3789–3791 (2004).
B. A. Trofimov and A. I. Mikhaleva, N-Vynilpyrroles, Nauka, Novosibirsk (1984).
B. A. Trofimov, in: The Chemistry of Heterocyclic Compounds, Part 2, Vol. 48; Pyrroles, R. A. Jones (ed.) Wiley, New York (1992), pp. 131–298.
Two-Step Synthesis of Pyrroles from Ketones and Acetylenes Through the Trofimov reaction, A. I. Mikhaleva and E. Yu. Schmidt, Selected Methods for Synthesis and Modification of Heterocycles, V. G. Kartsev (ed.), 1, 334–352, IBS Press, Moscow (2002).
A. B. Zaitsev, A. M. Vasil’tsov, E. Yu. Shmidt, et al., Zh. Org. Khim., 39, No. 10, 1479–1483 (2003).
H. Z. Chen, Y. D. Jin, R. S. Xu, et al., Synth. Met., 139, 529–534 (2003).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 03, Revision B. 03, Gaussian, Pittsburgh PA (2003).
F. H. Herbstein, M. Kapon, and G. M. Reisner, Acta Crystallogr., Sect. B: Struct. Sci., 42, 181 (1986); J. P. Reboul, Y. Oddon, C. Caranoni, et al., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 43, 537 (1987).
K. Moris and J. Laane, J. Mol. Struct., 413/414, 1313–1320 (1997); M. Takanashi, R. Ogino, and Y. Udagawa, Chem. Phys. Lett., 288, 714–718 (1998).
A. Dreiding, Angew. Chem., 76, 501/502 (1964).
V. A. Pal’m, An Introduction to Theoretical Organic Chemistry [in Russian], Vysshaya Shkola, Moscow (1974).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2006 by A. V. Vashchenko, A. M. Vasil’tsov, E. Yu. Shmidt, A. I. Mikhaleva, and B. A. Trofimov
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 47, No. 4, pp. 636–641, May–June, 2006.
Rights and permissions
About this article
Cite this article
Vashchenko, A.V., Vasil’tsov, A.M., Shmidt, E.Y. et al. The structure of dihydropyrroloindoles: A quantum-chemical estimation of conjugation in cyclohexadiene systems. J Struct Chem 47, 616–621 (2006). https://doi.org/10.1007/s10947-006-0347-z
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0347-z