Abstract
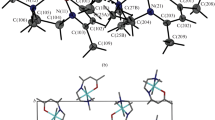
A complex salt of tetraammineplatinum(II) perrhenate [Pt(NH3)4](ReO4)2 has been synthesized. Crystal data: a = 5.1847(6) Å, b = 7.7397(8) Å, c = 7.9540(9) Å, α = 69.531(3)°, β = 79.656(3)°, γ = 77.649(3)°, V = 290,19(6) Å3, space group \(P\bar 1\), Z = 1, d x = 4.370 g/cm3. The products of decomposition of [Pt(NH3)4](ReO4)2 in a hydrogen atmosphere were investigated using X-ray phase analysis. In definite temperature modes, these are Pt0.33Re0.67 solid solutions based on Re with hcp cell parameters a = 2.76 Å, c = 4.42 Å. The products of thermolysis obtained from other precursor complex salts containing both Pt and Re were investigated. Thus Pt0.75Re0.25 solid solution with a = 3.905(3) Å was obtained from (NH4)2[ReCl6]0.25[PtCl6]0.75.
Similar content being viewed by others
References
P. S. Rudman, J. Less-Common Met., 12, 79–81 (1967).
V. Trzebyatowski and I. Berak, Izv. Polsk. Akad. Nauk., 2, No. 1, 37 (1954).
E. M. Savitskii, M. A. Tylkina, and K. B. Povarova, Rhenium Alloys [in Russian], Nauka, Moscow (1965).
A. I. Gubanov, “Binary complexes with tetraammine cations: precursors of metal powders,” Chemical Sciences Candidate’s Dissertation, Institute of Inorganic Chemistry, Siberian Division, Russian Academy of Sciences, Novosibirsk (2001).
I. V. Korolkov, A. I. Gubanov, and S. A. Gromilov, Zh. Strukt. Khim., 46, No. 3, 492–500 (2005).
S. A. Gromilov, S. V. Korenev, I. V. Korolkov, et al., ibid., 45, No. 3, 508–515 (2004).
I. I. Chernyaev, Synthesis of Complex Compounds from Platinum Metal Group [in Russian], Nauka, Moscow (1964).
Powder Diffraction File. Alphabetical Index. Inorganic Phases, JCPDS, International Center for Diffraction Data, PA, USA (1983).
R. J. Wasilewski, Trans. Metallurg. Soc. AIME, 221, 1081/1082 (1961).
W. Kraus and G. Nolze, J. Appl. Crystallogr., 9, 301–303 (1996).
G. M. Sheldrick, SHELXS-97 and SHELXL. Program for Refinement of Crystal Structure, Univ. Göttingen, Germany (1997).
A. C. Larson and R. B. Von Dreele, General Structure Analysis System, Los Alamos National Laboratory Report LAUR (2000), pp. 86–748.
S. I. Pechenyuk, V. Ya. Kuznetsov, R. A. Popova, et al., Zh. Neorg. Khim., 24, No. 12, 3306–3308.
F. D. Rochon, P. C. Kong, and R. Melanson, Acta Crystallogr. C, 46, 8–10 (1990).
V. A. Ushakov, E. M. Moroz, K. G. Richter, et al., Dokl. Akad. Nauk SSSR, 219, No. 1, 158–161 (1974).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2006 by I. V. Korolkov, A. V. Zadesenets, S. A. Gromilov, K. V. Yusenko, I. A. Baidina, and S. V. Korenev
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 47, No. 3, pp. 503–511, May–June, 2006.
Rights and permissions
About this article
Cite this article
Korolkov, I.V., Zadesenets, A.V., Gromilov, S.A. et al. Metal solid solutions obtained by thermolysis of Pt and Re salts. Crystal structure of [Pt(NH3)4](ReO4)2 . J Struct Chem 47, 489–498 (2006). https://doi.org/10.1007/s10947-006-0327-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0327-3