Abstract
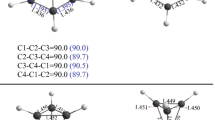
The geometrical and basic energy parameters of monosubstituted cumulenes and their singly and doubly charged cations were calculated by the Hartree-Fock and density functional (DFT) methods at a B3LYP level of theory using the 6-31G(d) basis set. The substituent was fluorine, cyan, amino group, phenyl, cyanophenyl, aminophenyl, or dimethylaminophenyl. In extended linear carbon systems based on cumulene, rotation of a terminal fragment depends on the character of the highest occupied molecular orbital (HOMO) from which electrons are removed. The terminal group rotates through 90 only when the contribution of electron density from the π molecular orbital (MO) of unsubstituted cumulene to the HOMO of substituted cumulene is over 70%. Otherwise, the terminal group rotates through a smaller angle; with a contribution of less than 30%, the dication is planar in any substituted cumulene. Thus quantitative criteria have been determined to evaluate the specific structural effect due to ionization of substituted cumulenes.
Similar content being viewed by others
References
J. Morley, J. Phys. Chem., 99, 10166–10174 (1995).
J. Y. Lee, B. J. Mhin, S. Mukame, and K. S. Kim, ibid., 119, 7519–7524 (2003).
H. Ding, T. W. Schmidt, T. Pino, et al., ibid., 814–819.
M. Weimer, W. Hieringer, F. Della Sala, and A. Görling, Chem. Phys., 309, No. 1, 77–87 (2005).
V. Bodart, J. Delhalle, M. Dory, and J.-M. André, J. Opt. Soc. Am. B, 4, 1047–1054 (1987).
B. Bildstein, O. Loza, and Yu. Chizhov, Organometal., 23, 1825–1835 (2004).
A. V. Belikov, Yu. E. Lozovik, A. G. Nikolaev, and A. M. Popov, Chem. Phys. Lett. A, 385, 72–78 (2004).
Yu. E. Lozovik, A. V. Minogin, and A. M. Popov, Phys. Lett. A, 313, 112–121 (2003).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 03, Gaussian Inc., Pittsburgh, PA (2003).
M. van Meerssche, G. Germain, J. P. Declercq, et al., Acta Crystallogr., Sect. B, 33, 3873–3875 (1977).
H. Irngartinger and W. Gotzmann, Angew. Chem., Int. Ed. Engl., 25, 340–342 (1986).
W. Skibar, H. Kopacka, K. Wurst, et al., Organometal., 23, 1024–1041 (2004).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2006 by O. Yu. Podkopaeva and Yu. V. Chizhov
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 47, No. 3, pp. 436–442, May–June, 2006.
Rights and permissions
About this article
Cite this article
Podkopaeva, O.Y., Chizhov, Y.V. DFT study of the geometrical and electronic structure of substituted cumulenes in netral and cationic forms. J Struct Chem 47, 420–426 (2006). https://doi.org/10.1007/s10947-006-0317-5
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0317-5