Abstract
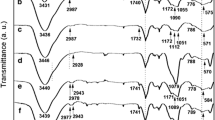
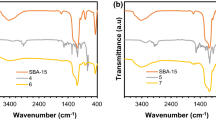
Mesoporous helical silica were prepared by doping of chiral diammoniumcyclohexane mono-tartrate salt in a sol–gel process, then functionalized to immobilize Mn(III)-salen complexes for oxidative kinetic resolution of secondary alcohols. Characterization revealed the doped silica significantly changed chiral recognition, porosity and hydrolysis resistance compared with blank-doped one. In catalysis, helical silica-supported Mn(III)-salen showed satisfactory conversion, enantioselectivity and selectivity factor, while major configuration of resolution products was determined by configuration of silica supports more than Mn(III)-salen. Furthermore, linkage of (S,S)-diammonium salt-doped silica with Mn(III)-(R,R)salen showed better enantioselectivity than other combinations. At last, in addition to iodobenzene diacetate, hydrogen peroxide (30 wt%) was another effective terminal oxidant. This work not only provided new chiral silica materials, but also explored their application in catalytic resolution.








Similar content being viewed by others
References
A. Lumbroso, M.L. Cooke, B. Breit, Angew. Chem. Int. Ed. 52, 1890–1932 (2013)
T. Shibata, K. Iwahashi, T. Kawasaki, K. Soai, Tetrahedron Asymmetry 18, 1759–1762 (2007)
M. Wang, Y. Wang, G. Li, P. Sun, J. Tian, H. Lu, Tetrahedron Asymmetry 22, 761–768 (2011)
K. Huang, X. Zhang, H. Geng, S. Li, X. Zhang, ACS Catal. 2, 1343–1345 (2012)
A. Ghanem, H.Y. Aboul-Enein, Chirality 17, 1–15 (2005)
K. Murakami, Y. Sasano, M. Tomizawa, M. Shibuya, E. Kwon, Y. Iwabuchi, J. Am. Chem. Soc. 136, 17591–17600 (2014)
K. Masutani, T. Uchida, R. Irie, T. Katsuki, Tetrahedron Lett. 41, 5119–5123 (2000)
W. Sun, H. Wang, C. Xia, J. Li, P. Zhao, Angew. Chem. Int. Ed. 42, 1042–1044 (2003)
S. Sahoo, P. Kumar, F. Lefebvre, S.B. Halligudi, Tetrahedron Lett. 49, 4865–4868 (2008)
P.K. Bera, N.C. Maity, S.H.R. Abdi, N.H. Khan, R.I. Kureshy, H.C. Bajaj, App. Catal. A 467, 542–551 (2013)
R. Tan, Y. Dong, M. Peng, W. Zheng, D. Yin, App. Catal. A 458, 1–10 (2013)
H. Mizoguchi, T. Uchida, T. Katsuki, Angew. Chem. Int. Ed. 126, 3242–3246 (2014)
O. Långvik, D. Mavrynsky, R. Leino, Catal. Today 241, 255–259 (2015)
I. Fechete, Y. Wang, J.C. Védrine, Catal. Today 189, 2–27 (2012)
F. Hoffmann, M. Cornelius, J. Morell, M. Fröba, Angew. Chem. Int. Ed. 45, 3216–3251 (2006)
T. Lu, X. Yao, M.G.Q. Lu, Y. He, J. Porous Mater. 17, 123–131 (2010)
S. Che, Z. Liu, T. Ohsuna, K. Sakamoto, O. Terasaki, T. Tatsumi, Nature 429, 281–284 (2004)
Y. Shang, Y. Li, X. He, S. Du, L. Zhang, E. Shi, S. Wu, Z. Li, P. Li, J. Wei, K. Wang, H. Zhu, D. Wu, A. Cao, ACS Nano 7, 1446–1453 (2013)
C.I. Fernandes, M.S. Saraiva, T.G. Nunes, P.D. Vaz, C.D. Nunes, J. Catal. 309, 21–32 (2014)
R.A. Shelton, I.W.C.E. Arends, G.T. Brink, A. Dijksman, Acc. Chem. Res. 35, 774–781 (2002)
G. Wu, X. Wang, J. Li, N. Zhao, W. Wei, Y. Sun, Catal. Today 131, 402–407 (2008)
J.F. Larrow, E.N. Jacobsen, Y. Gao, Y. Hong, X. Nie, C.M. Zepp, J. Org. Chem. 59, 1939–1942 (1994)
Z. Zhang, F. Guan, X. Huang, Y. Wang, Y. Sun, J. Mol. Catal. A: Chem. 363–364, 343–353 (2012)
Z. Guo, Y. Du, Y. Chen, S.-C. Ng, Y. Yang, J. Phys. Chem. C 114, 14353–14361 (2010)
Y. Han, L. Zhao, J.Y. Ying, Adv. Mater. 19, 2454–2459 (2007)
H. Zhang, Y. Zhang, C. Li, J. Catal. 238, 369–381 (2006)
B.M. Choudary, T. Ramani, H. Maheswaran, L. Prashant, K.V.S. Ranganath, K.V. Kumar, Adv. Synth. Catal. 348, 493–498 (2006)
S.P. Newman, W. Jones, J. Solid State Chem. 148, 26–40 (1999)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquérol, T. Siemieniewska, Pure Appl. Chem. 57, 603–619 (1985)
A. Galarneau, M. Nader, F. Guenneau, F.D. Renzo, A. Gedeon, J. Phys. Chem. C 111, 8268–8277 (2007)
R. Fu, N. Yoshizawa, M.S. Dresselhaus, G. Dresselhaus, J.H. Satcher Jr, T.F. Baumann, Langmuir 18, 10100–10104 (2002)
Z. Zhao, M. Li, J. Zhang, H. Li, P. Zhu, W. Liu, Ind. Eng. Chem. Res. 51, 9531–9539 (2012)
N. Andreu, D. Flahaut, R. Dedryvère, M. Minvielle, H. Martinez, D. Gonbeau, A.C.S. Appl, Mater. Interfaces 7, 6629–6636 (2015)
Y. Snir, R.D. Kamien, Science 307, 1067 (2005)
R.I. Kureshy, I. Ahmad, K. Pathak, N.H. Khan, S.H.R. Abdi, J.K. Prathap, R.V. Jasra, Chirality 19, 352–357 (2007)
Z. Li, Z.H. Tang, X.X. Hu, C.G. Xia, Chem. Eur. J. 11, 1210–1216 (2005)
K. Yu, Z. Gu, R. Ji, L. Lou, F. Ding, C. Zhang, S. Liu, J. Catal. 252, 312–320 (2007)
E.M. McGarrigle, D.G. Gilheany, Chem. Rev. 105, 1563–1602 (2005)
Acknowledgments
This work was supported by Shaanxi Higher Education Teaching Reform Project (No. 13BY02, Cultivation of Creative Ability of Scientific Research for the Undergraduate), and the Fundamental Research Funds for the Central Universities (No. xjj2014005, Application of Porous Helical Support in Catalytic Asymmetric Reactions).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, L., Li, L., Li, Y. et al. Mesoporous helical silica immobilizing manganese(III)-salen complex for oxidative kinetic resolution of secondary alcohols. J Porous Mater 23, 19–33 (2016). https://doi.org/10.1007/s10934-015-0052-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-0052-4