Abstract
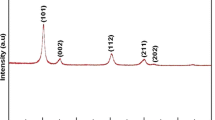
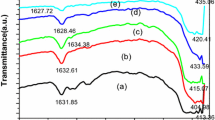
One of ETS-10 variants, aluminum-substituted ETAS-10 was successfully synthesized with Al/Ti molar ratio of 0.1–0.4 using different titanium sources, titanium sulfate (Ti(SO4)2) and titanium oxysulfate (TiOSO4). Through the compositional study, like ETS-10, the (Na + K)/Na molar ratio significantly affects the crystallinity and phase purity depending on titanium source. The 23 factorial designs indicate that the high alkalinity mainly by Na+ content is very critical to the crystallization of pure ETAS-10 while the content of K+ cation should be carefully controlled. It suggests that the increase in K+ content with smaller hydrated radius than of Na+ makes the formation of ETAS-10 structure difficult. The optimum composition was chosen and applied to kinetic study. The activation energies for three different stages, nucleation, transition and crystallization were calculated using the modified Avrami–Erofeev equation. This result indicates that the nucleation and transition stage proceed at the similar rate, and then the crystallization stage proceeds with lower activation energy than those of previous two stages. In addition, the temperature dependency on the crystallization was quite significant, favoring high temperature.





Similar content being viewed by others
References
S.M. Kuznicki, US Patent. 4,853,202, 1989
S.M. Kuznicki, US Patent 4,938,939, 1990
S.M. Kuznicki, EU Patent 0,457,988A1, 1991
L. Al-Attar, A. Dyer, R. Blackburn, J. Radioanal. Nucl. Chem. 246, 451 (2000)
L. Al-Attar, A. Dyer, A. Pajanen, R. Harjula, J. Mater. Chem. 13, 2969 (2003)
C.C. Pavel, D. Vuono, A. Nastro, J.B. Nagy, N. Bilba, Stud. Surf. Sci. Catal. 142, 295 (2002)
S.M. Kuznicki, R.J. Madon, G.S. Koermer, K.A. Thrush, Euro Patent 0,405,978A1, 1991
S.M. Kuznicki, A.K. Thrush, WO 91/18833, 1991
M.W. Anderson, J. Rocha, Z. Lin, A. Philippou, I. Orion, A. Ferreira, Micropor. Mater. 6, 195 (1996)
J. Rocha, Z. Lin, A. Ferreira, M.W. Anderson, J. Chem. Soc. Chem. Commun., 867 (1995)
Z. Lin, J. Rocha, A. Ferreira, M.W. Anderson, Coll. Surf. 179, 133 (2001)
L. Lv, F. Su, X.S. Zhao, Micropor. Mesopor. Mater. 101, 355 (2007)
J. Rocha, A. Ferreira, Z. Lin, M.W. Anderson, Micropor. Mesopor. Mater. 23, 253 (1998)
S.D. Kim, S.H. Noh, K.H. Seong, W.J. Kim, Micropor. Mesopor. Mater. 72, 185 (2004)
W.J. Kim, M.C. Lee, J.C. Yoo, D.T. Hayhurst, Micropor. Mesopor. Mater. 41, 79 (2000)
S.H. Noh, S.D. Kim, Y.J. Chung, J.W. Park, D.K. Moon, D.T. Hayhurst, W.J. Kim, Micropor. Mesopor. Mater. 88, 197 (2006)
W.J. Kim, S.D. Kim, H.S. Jung, D.T. Hayhurst, Micropor. Mesopor. Mater. 56, 89 (2002)
M.W. Anderson, A. Philippou, Z. Lin, A. Ferreira, J. Rocha, Angew. Chem. Int. Ed. Engl. 34, 1003 (1995)
Acknowledgement
This research program was financially supported through General Research Grant No. R01-2007-000-20364-0 of KOSEF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.D., Noh, S.H., Kim, Y.C. et al. Hydrothermal synthesis of aluminum-substituted titanosilicate, ETS-10. J Porous Mater 16, 307–314 (2009). https://doi.org/10.1007/s10934-008-9201-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-008-9201-3