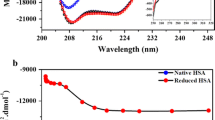
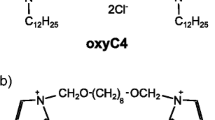
Abstract
Gemini surfactants are a new class of surfactants that consist of two hydrophilic head groups and two hydrophobic tails separated by a spacer group. As the properties of geminis are different to their monomeric counterparts, a large number of applications have been investigated. Here we report on the use of a new class of gemini detergents containing a disulfide bond in the spacer (Det-SS-Det) for protein refolding. Using lysozyme as a model protein we could demonstrate that the disulfide gemini detergents allow oxidative refolding of the protein in the absence of any external redox system in an “artificial chaperone system”. Refolding kinetics using gemini disulfide detergents differing in their hydrophobicity were analysed to determine the folding and aggregation rate constants. The results point to an important role of the transiently formed mixed disulfides between the protein and the detergent (Prot-SS-Det) in the oxidative refolding process of lysozyme.








Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- ALP:
-
Alkaline phosphatase
- β-CD:
-
β-cyclodextrin
- BSA:
-
Bovine serum albumin
- CD:
-
Cyclodextrin
- CMC:
-
Critical micelle concentration
- CTAB:
-
Cetyltrimethylammonium bromide
- C9SS:
-
Nonyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- C10SS:
-
Decyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- C11SS:
-
Undecyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- C12SS:
-
Dodecyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- C13SS:
-
Tridecyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- C14SS:
-
Tetradecyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- C15SS:
-
Pentadecyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- C16SS:
-
Hexadecyl-dimethyl-(2-sulfanylethyl)azanium-disulfide
- DTAB:
-
Dodecyltrimethylammonium bromide
- DTTox:
-
Oxidized dithiothreitol
- DTTred:
-
Reduced dithiothreitol
- E. coli:
-
Escherichia coli
- EDTA:
-
Ethylenediaminetetraacetic acid
- GdmCl:
-
Guanidine hydrochloride
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- HPLC:
-
High pressure liquid chromatography
- IB:
-
Inclusion body
- m-β-CD:
-
Methyl-β-cyclodextrin
- RNAse A:
-
Ribonuclease A
- RP-HPLC:
-
Reversed phase high pressure liquid chromatography
- RT:
-
Room temperature
- TCEP:
-
Tris (2-carboxyethyl) phosphine hydrochloride
- TFA:
-
Trifluoroacetic acid
- Tris:
-
Tris (hydroxymethyl)-aminomethan
- TTAB:
-
Tetradecyltrimethylammonium bromide
References
Aachmann FL, Otzen DE, Larsen KL, Wimmer R (2003) Protein Eng 16:905–912
Beld J, Woycechowsky KJ, Hilvert D (2008) Biochemistry 47:6985–6987
Cleland JL, Randolph TW (1992) J Biol Chem 267:3147–3153
Couthon F, Clottes E, Vial C (1996) Biochem Biophys Res Commun 227:854–860
De Bernardez Clark E, Hevehan D, Szela S, Maachupalli-Reddy J (1998) Biotechnol Prog 14:47–54
Dong XY, Huang Y, Sun Y (2004) J Biotechnol 114:135–142
Dong XY, Shi JH, Sun Y (2002) Biotechnol Prog 18:663–665
Dong XY, Wang YB, Liu X, Sun Y (2001) Biotechnol Lett 23:1165–1169
Gough JD, Williams RH, Donofrio AE, Lees WJ (2002) J Am Chem Soc 124:3885–3892
Guerrero-Martinez A, Gonzalez-Gaitano G, Vinas MH, Tardajos G (2006) J Phys Chem 110:13819–13828
Gull N, Sen P, Khan RH, Kabir-ud-Din (2009) J Biochem 145:67–77
Hagihara Y, Aimoto S, Fink AL, Goto Y (1993) J Mol Biol 231:180–184
Heerklotz H, Epand RM (2001) Biophys J 80:271–279
Hevehan DL, De Bernardez Clark E (1997) Biotechnol Bioeng 54:221–230
Khodagholi F, Eftekharzadeh B, Yazdanparast R (2008) Protein J 27:123–129
Khodagholi F, Yazdanparast R, Sadeghirizi A (2007) J Biomol Struct Dyn 25:189–194
Khodarahmi R, Yazdanparast R (2005) Int J Biol Macromol 36:191–197
Loibner H, Pruckner A, Stütz A (1984) Tetrahedron Lett 25:2535–2536
Maachupalli-Reddy J, Kelley BD, De Bernardez Clark E (1997) Biotechnol Prog 13:144–150
Madar DJ, Patel AS, Lees WJ (2009) J Biotechnol 142:214–219
Marston FAO (1986) Biochem J 240:1–12
Menger FM, Littau CA (1991) J Am Chem Soc 113:1451–1452
Mwakibete H, Bloor DM, Wyn-Jones E (1994) Langmuir 10:3328–3331
Ouyang M, Remy JS, Szoka FC (2000) Bioconjugate Chem 11:104–112
Perraudin JP, Torchia TE, Wetlaufer DB (1983) J Biol Chem 258:11834–11839
Pi Y, Shang Y, Peng C, Liu H, Hu Y, Jiang J (2006) Biopolymers 83:243–249
Prabha CR, Rao ChM (2004) Fed Europ Biochem Soc 557:69–72
Qoronfleh MW, Hesterberg LK, Seefeldt MB (2007) Protein Expression and Purif 55:209–224
Raman B, Ramakrishna T, Rao CM (1996) J Biol Chem 271:17067–17072
Roux P, Ruoppolo M, Chaffotte A, Goldberg ME (1999) Protein Sci 8:2751–2760
Rozema D, Gellman SH (1996) Biochemistry 35:15760–15771
Sharp TR, Rosenberry TL (1982) J Biochem Bioph Methods 6:159–172
Wain R, Smith LJ, Dobson CM (2005) J Mol Biol 351:662–671
Weissman JS, Hohl CM, Kovalenko O, Kashi Y, Chen S, Braig K, Saibil HR, Fenton WA, Horwich AL (1995) Cell 83:577–587
Wetlaufer DB, Branca PA, Chen G (1987) Protein Eng 1:141–146
Wetlaufer DB, Saxena VP (1970) Biochemistry 9:5015–5023
Yamaguchi S, Yamamoto E, Tsukiji S, Nagamune T (2008) Biotechnol Prog 24:402–408
Yazdanparast R, Esmaeili MA, Khodagholi F (2007) Int J Biol Macromol 40:126–133
Yazdanparast R, Khodarahmi R, Soori E (2005) Arch Biochem Biophys 437:178–185
Yazdanparast R, Khodagholi F (2006) Arch Biochem Biophys 446:11–19
Zana R, Talmon Y (1993) Nature 362:228–230
Acknowledgments
This work was supported by the Landesstiftung Baden-Württemberg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Potempa, M., Hafner, M. & Frech, C. Mechanism of Gemini Disulfide Detergent Mediated Oxidative Refolding of Lysozyme in a New Artificial Chaperone System. Protein J 29, 457–465 (2010). https://doi.org/10.1007/s10930-010-9279-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9279-8