Abstract
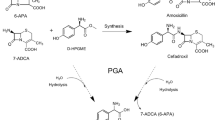
Penicillin amidase from Alacaligenes faecalis is an attractive biocatalyst for hydrolysis of penicillin G for production of 6-aminopenicillanic acid, which is used in the synthesis of semi-synthetic β-lactam antibiotics. Recently a mutant of this enzyme with extended C-terminus of the A-chain comprising parts of the connecting linker peptide was constructed. Its turnover number for the hydrolysis of penicillin G was 140 s−1, about twice of the value for the wild-type enzyme (80 s−1). At the same time the specificity constant was improved about three-fold. The wild-type and the mutant enzymes showed similar pH stability suggesting that the linker peptide fragment covalently attached to the A-chain does not alter the electrostatic interactions in the protein core. Although the global stability of A. faecalis wild-type enzyme and the T206GS213G variant does not differ, the presence of the linker fragment stabilizes the domains interface, as evidenced by the monophasic transition of the mutant enzyme from folded to unfolded state during urea-induced denaturation. The high stability and activity of the mutant enzyme provides a rationale to use it as a biocatalyst in the industrial processes, where the enzyme must be more robust to fluctuations in the operational conditions.





Similar content being viewed by others
Abbreviations
- ANS:
-
8-Anilino-1-naphtalenesulfonic acid
- DTT:
-
Dithiotreitol
- NIPAB:
-
6-Nitro-3-(phenylacetamido)benzoic acid
- PA:
-
Penicillin amidase
References
Arroyo M, de la Mata I, Acebal C, Pilar Castillon M (2003) Appl Microbiol Biotechnol 60:507–514
Brannigan JA, Dodson G, Duggleby HJ, Moody PC, Smith JL, Tomchik DR, Murzin AG (1995) Nature 378:416–419
Bruggink A, Roos EC, de Vroom E (1998) Org Proc Res Dev 2:128–133
Buchholz K, Kasche V, Bornscheuer UT (2005) Basics of enzymes as biocatalysts. In: Biocatalysts and enzyme technology. Wiley-VCH, Weinheim, pp 102, 359
Chrunyk BA, Evans J, Lillquist J, Young P, Wetze R (1993) J Biol Chem 268:18053–18061
Clark PL, Weston BF, Gierasch LM (1998) Fold Des 3:401–412
Duggleby HJ, Tolley SP, Hill CP, Dodson EJ, Dodson GG, Moody CE (1995) Nature 373:264–268
Galunsky B, Lummer K, Kasche V (2000) Monatsh Chem 131:623–632
Galunsky B, Kasche V (2002) Adv Synth Catal 344:1115–1119
Guranda DT, Volovik TS, Svedas VK (2004) Biochem Mosc 69:1386–1390
Hewitt L, Kasche V, Lummer K, Lewis RJ, Murshudov GN, Verma CS, Dodson GG, Wilson KS (2000) J Mol Biol 302:887–898
Ignatova Z, Stoeva S, Galunsky B, Hörnle C, Nurk A, Piotraschke E, Voelter W, Kasche V (1998) Biotechnol Lett 20:977–982
Ignatova Z (2000) PhD thesis Technische Universität Hamburg-Harburg
Ignatova Z, Enfors S, Hobbie M, Taruttis S, Vogd C, Kasche V (2000) Enzyme Microb Technol 26:165–170
Ignatova Z, Hörnle C, Nurk A, Kasche V (2002) Biochem Biophys Res Commun 291:146–149
Ignatova Z, Wischnewski F, Notbohm H, Kasche V (2005) J Mol Biol 348:999–1014
Jaenicke R (1999) Prog Biophys Mol Biol 71:155–241
Jiang X, Smith CS, Petrassi MH, Hammarström P, White JT, Sacchettini JC, Kelly JW (2001) Biochemistry 40:11442–11452
Kasche V (1986) Enzyme Microb Technol 8:4–16
Kasche V, Lummer K, Nurk A, Piotraschke E, Rieks A, Stoeva S, Voelter W (1999) Biochim Biophys Acta 1433:76–86
Kasche V, Galunsky B, Ignatova Z (2003) Eur J Biochem 270:4721–4728
Kasche V, Ignatova Z, Märkl H, Plate W, Punckt N, Schmidt D, Wiegandt K, Ernst B (2005) Biotechnol Prog 21:432–438
Khurana R, Gillespie JR, Talapatra A, Minert LJ, Ionescu-Zanetti C, Millett I, Fink AL (2001) Biochemistry 40:3525–3535
Lummer K (2000) PhD thesis Technische Universität Hamburg-Harburg
McVey CE, Walsh MA, Dodson GG, Wilson KS, Brannigan JA (2001) J Mol Biol 313:139–150
Pace NC, Scholtz MJ (1997) Measuring the conformational stability of a protein. In: Creighton TE (ed) Protein structure: a practical approach. IRL Press, Oxford, pp 299–321
Privalov PL (1996) J Mol Biol 258:707–725
Quintas A, Saraiva MJM, Brito RMM (1999) J Biol Chem 274:32943–32949
Svedas V, Guranda D, van Langen L, van Rantwijk F, Sheldon R (1997) FEBS Lett 417:414–418
Svedas VK, Margolin AL, Sherestyuk CF, Klyosov AA, Berezin IV (1977) Biotechnol Lett 7:877–882
Verhaert RMD, Riemens AM, van der Laan JM, van Duin J, Quax WJ (1997) Appl Environ Microbiol 63:3412–3418
Vogl T, Brengelmann R, Hinz HJ, Scharf M, Lötzbeyer M, Engels JW (1995) J Mol Biol 254:481–496
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuryev, R., Kasche, V., Ignatova, Z. et al. Improved A. faecalis Penicillin Amidase Mutant Retains the Thermodynamic and pH Stability of the Wild Type Enzyme. Protein J 29, 181–187 (2010). https://doi.org/10.1007/s10930-010-9238-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9238-4