Abstract
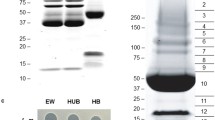
Exposure of selected Gram-positive and Gram-negative bacterial pathogens to egg shell membranes (ESM) significantly reduced their thermal resistance and/or inactivated cells. Although the components responsible for this antibacterial activity have not been conclusively identified, several proteins associated with the ESM activity have been identified including β-N-acetylglucosaminidase, lysozyme and ovotransferrin, with each displaying varying degrees of antibacterial activity. Numerous attempts to purify active fractions of β-N-acetylglucosaminidase, lysozyme and ovotransferrin from the ESM proved somewhat limited; however, hen egg white (HEW) β-N-acetylglucosaminidase was purified using a two-step chromatographic procedure, isoelectric focusing followed by cation exchange chromatography. Pure fractions of ovotransferrin were also obtained in the process. SDS-PAGE electrophoresis and Matrix-Assisted Laser Desorption Time-of-Flight Mass Spectrometry were then used to partially characterize the individual protein components. Purified protein fractions such as these will be required in order to fully elucidate the mechanism responsible for the antimicrobial properties associated with the ESM.
Similar content being viewed by others
Abbreviations
- β-NAGase:
-
β-N-acetylglucosaminidase
- ESM:
-
eggshell membrane
- HEW:
-
hen egg white
- WL:
-
White Leghorn
- P-9-L:
-
polyethylene 9-lauryl sulfate
- GauHCl:
-
guanadine HCl
- SDS:
-
sodium dodecyl sulfate
- LDS:
-
lithium dodecyl sulfate
- BCA:
-
bicinchoninic acid
References
S. A. Al-Mashikhi S. Nakaï (1987) Agric. Biol. Chem. 51 2881–2887 Occurrence Handle1:CAS:528:DyaL1cXjsVartQ%3D%3D
G. Alderton W. H. Ward H. L. Fevold (1946) Arch. Biochem. Biophys. 11 9–15 Occurrence Handle1:CAS:528:DyaH28XjslClsA%3D%3D
G.J. Ahlborn B.W. Sheldon (2005) Poult. Sci. 84 1935–1941 Occurrence Handle1:CAS:528:DC%2BD2MXhtlamsLfJ
P. Azari R. F. Baugh (1967) Arch. Biochem. 118 138–144 Occurrence Handle10.1016/0003-9861(67)90289-5 Occurrence Handle1:CAS:528:DyaF2sXktlOnsg%3D%3D
J. R. Baker D. A. Balch (1962) J. Biochem. 82 352–361 Occurrence Handle1:CAS:528:DyaF38Xmt12isA%3D%3D
Board, R.G., and Tranter, H.S. (1994). In: Stadelman W.J., and Cotterill O. J (eds.), Egg Science and Technology, 4th ed. The Haworth Press, Inc. Binghamton, NY, pp. 81–104
C. M. Chung S. L. Chan S. Shimizu (1991) Int. J. Biochem. 23 609–616 Occurrence Handle1:CAS:528:DyaK3MXitVarsrk%3D
T. Croguennec F. Nau S. Pezennec M. Piot G. Brulé (2001) Eur. Food. Res. Technol. 212 296–301 Occurrence Handle10.1007/s002170000242 Occurrence Handle1:CAS:528:DC%2BD3MXjtFagsLg%3D
G. Crombie R. Snider B. Faris C. Franzbrau (1981) Biochim. Biophys. Acta. 640 365–367 Occurrence Handle1:CAS:528:DyaL3MXmtVWntQ%3D%3D
J. W. Donovan L. U. Hansen (1971) J. Food Sci. 36 174–177 Occurrence Handle1:CAS:528:DyaE3MXksVOls7Y%3D
L. E. Elliott A. W. Brant (1957) Food Res. 22 241–250
H. Fraenkel-Conrat R. E. Feeney (1950) Arch. Biochem. 29 101–113 Occurrence Handle1:CAS:528:DyaG3MXhsVSmsA%3D%3D
J. A. Garibaldi (1960) Food Res. 25 337–344
J. Gautron M. T. Hincke M. Panheleux J. M. Garcia-Riuz T. Boldicke Y. Nys (2001) Connect. Tissue Res. 42 255–267 Occurrence Handle1:CAS:528:DC%2BD38Xlt12gtr8%3D
C. Guerin G. Brule (1992) Sci. Aliment. 12 705–720 Occurrence Handle1:CAS:528:DyaK3sXhvFShtL8%3D
E. D. Harris J. E. Blount (1980) Science 208 55–56 Occurrence Handle1:CAS:528:DyaL3cXhvFCrt7k%3D
M. T. Hincke J. Gautron M. Panheleux J. Garcia-Ruiz M. D. McKee Y. Nys (2000) Matrix Biol. 19 443–453 Occurrence Handle10.1016/S0945-053X(00)00095-0 Occurrence Handle1:CAS:528:DC%2BD3cXnt1aqtLo%3D
Ibrahim, H. R. (1996). In: Hen Eggs: Their Basic and Applied Science, CRC Press, Inc., New York, pp. 37–57
J. M. Jeltsch P. Chambon (1982) Eur. J. Biochem. 122 291–295 Occurrence Handle10.1111/j.1432-1033.1982.tb05879.x Occurrence Handle1:CAS:528:DyaL38XhtlOjt70%3D
P. Jolles J. Jolles (1984) Mol. Cell. Biochem. 63 165–189 Occurrence Handle1:CAS:528:DyaL2cXmtVKnsb0%3D
T. Kato K. Hatanaka T. Mega S. Hase (1997) J. Biochem. 122 1167–1173 Occurrence Handle1:CAS:528:DyaK1cXmtFentw%3D%3D
U. K. Laemmli (1970) Nature 227 680–685 Occurrence Handle10.1038/227680a0 Occurrence Handle1:CAS:528:DC%2BD3MXlsFags7s%3D
A. Lifshitz R. C. Baker H. B. Naylor (1963) J. Food Sci. 28 94–99
I. E. Lush J. Conchie (1966) Biochem. Biophys. Acta. 130 81–86 Occurrence Handle1:CAS:528:DyaF2sXis1yntg%3D%3D
Y. Ogawa R. Nakamura (1983) Agric. Biol. Chem. 48 229–230
Y. Ogawa R. Nakamura Y. Sato (1983) Agric. Biol. Chem. 47 2085–2089 Occurrence Handle1:CAS:528:DyaL3sXlslWrs7g%3D
A. L. Poland B. W. Sheldon (2001) J. Food Prot. 64 486–492 Occurrence Handle1:CAS:528:DC%2BD3MXntVais78%3D
M. B. Rhodes P. R. Azari R. E. Feeney (1958) J. Biol. Chem. 230 399–408 Occurrence Handle1:CAS:528:DyaG1cXltFSqsw%3D%3D
D. S. Robinson N. R. King (1968) J. R. Microsc. Soc. 88 13–22 Occurrence Handle1:CAS:528:DyaF1cXktV2isrc%3D
D. Robinson J. L. Stirling (1968) Biochem. J. 107 321–327 Occurrence Handle1:CAS:528:DyaF1cXktVShsLg%3D
Simkiss, K. (1958). Ph.D. Thesis, University of Reading
B. C. Starcher S. G. King (1980) Connect. Tissue. Res. 8 53–55 Occurrence Handle1:CAS:528:DyaL3MXmt1WjsA%3D%3D Occurrence Handle10.3109/03008208009152122
G. D. Schockman J. V. Höltje (1994) NoChapterTitle M. J. Guysen R. Hakenbeck (Eds) New Comprehensive Biochemistry Elsevier Science Amsterdam 131–167
K. Takahashi K. Shirai M. Kitamura M. Hattori (1996) Biosci. Biotechnol. Biochem. 60 1299–1302 Occurrence Handle1:CAS:528:DyaK28Xltlektb0%3D Occurrence Handle10.1271/bbb.60.1299
D. V. Vadehra R. C. Baker H. B. Naylor (1972) Comp. Biochem. Physiol. 43 503–508 Occurrence Handle1:CAS:528:DyaE3sXhtlyrsA%3D%3D
P. Valenti P. Visca G. Antonini N. Orsi (1985) Mycopathologia 89 169–175 Occurrence Handle10.1007/BF00447027 Occurrence Handle1:STN:280:DyaL2M7ovVWqtg%3D%3D
P. Valenti P. Visca G. Antonini N. Orsi E. Antonini (1987) Med. Microbiol. Immunol. Berl. 176 123–130 Occurrence Handle1:CAS:528:DyaL2sXksFGls7g%3D
B. Weissmann S. Hadjiioannou J. Tornheim (1964) J. Biol. Chem. 239 59–63 Occurrence Handle1:CAS:528:DyaF2cXktlGq
S. E. Winn H. R. Ball SuffixJr. (1975) Poult. Sci. 54 799–805 Occurrence Handle1:CAS:528:DyaE2MXlt1yqtL8%3D
Winn, S. E., and Ball, H. R. Jr. (1996). Personal communication, North Carolina State University, Raleigh, N.C.
M. Wong M. J. Hendrix K. Mark Particlevon der C. Little R. Stern (1984) Dev. Biol. 104 28–36 Occurrence Handle10.1016/0012-1606(84)90033-2 Occurrence Handle1:CAS:528:DyaL2cXksFajtb8%3D
F. Yi Z. X. Guo L. X. Zhang J. Yu Q. Li (2004) Biomaterials 25 4591–4599 Occurrence Handle10.1016/j.biomaterials.2003.11.052 Occurrence Handle1:CAS:528:DC%2BD2cXjsFOgurs%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahlborn, G.J., Clare, D.A., Sheldon, B.W. et al. Identification of Eggshell Membrane Proteins and Purification of Ovotransferrin and β-NAGase from Hen Egg White. Protein J 25, 71–81 (2006). https://doi.org/10.1007/s10930-006-0010-8
Issue Date:
DOI: https://doi.org/10.1007/s10930-006-0010-8