Abstract
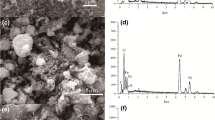
The present study reported the synthesis of novel organic–inorganic hybrid nanocomposite by incorporating tin (IV) based ion exchanger into the hybrid polymer network of chitosan and gelatin prepared under vacuum for the efficient removal of heavy metal ions and toxic dyes from an aqueous fluid. The physicochemical studies such as ion exchange capacity (IEC), chemical stability, thermal stability, pH titration and distribution behaviour studies were also carried out to determine the cation exchange behaviour of the material. The surface morphology and structural properties were studied by the techniques such as FTIR, FESEM, EDS, TEM and XRD. Distribution studies confirmed the synthesized CG/STPNC had the highest selectivity for Pb2+ ions (85.3 mL/g). Maximum adsorption of methylene blue (82%) was achieved within 240 min at 500 mg of adsorbent dose, 10 mg/L of the initial concentration of dye, pH of 7 and 30 °C of temperature. Adsorption kinetic data fitted well with pseudo-second order rate model with R2 = 0.995. The correlation value 0.95 and favourable RL = 0.21 of adsorption data suggested better fit for Langmuir adsorption. Thus the synthesized nanocomposite ion exchanger was found to be a promising cation exchanger as well as an adsorbent for heavy metal ion and dye removal from textile industrial effluents.
Graphical abstract









Similar content being viewed by others
Abbreviations
- C:
-
Chitosan
- G:
-
Gelatin
- HPN:
-
Hybrid polymer network
- GLA:
-
Gluteraldehyde
- IEC:
-
Ion exchange capacity
- CG/STPNC:
-
Chitosan–gelatin/Sn(iv)tungstatophosphate nanocomposite
- STP:
-
Sn (IV) tungstatophosphate
- MB:
-
Methylene blue
References
Hui KS, Chao CYH, Kot SC (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 127:89–101. https://doi.org/10.1016/j.jhazmat.2005.06.027
Ayhan S (2008) Adsorption of malachite green onto bentonite: equilibrium and kinetic studies and process design. 115:234–246. https://doi.org/10.1016/j.micromeso.2008.01.039
Sharma J, Chadha AS, Pruthi V et al (2017) Sequestration of dyes from artificially prepared textile effluent using RSM-CCD optimized hybrid backbone based adsorbent-kinetic and equilibrium studies. J Environ Manag 190:176–187. https://doi.org/10.1016/j.jenvman.2016.12.065
Shanker U, Rani M, Jassal V (2017) Degradation of hazardous organic dyes in water by nanomaterials. Environ Chem Lett 15:623–642. https://doi.org/10.1007/s10311-017-0650-2
Amini M, Ashrafi M (2016) Photocatalytic degradation of some organic dyes under solar light irradiation using TiO2 and ZnO nanoparticles. Nano Chem Res 1:79–86. https://doi.org/10.7508/ncr.2016.01.010
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Issazadeh H, Engineering G (2015) Preparation of new conductive polymer nanocomposites for cadmium removal from industrial wastewaters Leila Zoleikani, Hossein Issazadeh, Bahman ZareNezhad. 71–80
Lee SB, Ha DI, Cho SK et al (2003) Temperature/pH-sensitive comb-type graft hydrogels composed of chitosan and poly (N-isopropylacrylamide)
Sharma J, Anand P, Pruthi V et al (2017) RSM-CCD optimized adsorbent for the sequestration of carcinogenic rhodamine-B: kinetics and equilibrium studies. Mater Chem Phys 196:270–283. https://doi.org/10.1016/j.matchemphys.2017.04.042
Vermeer AWP, McCulloch JK, Van Riemsdijk WH, Koopal LK (1999) Metal ion adsorption to complexes of humic acid and metal oxides: deviations from the additivity rule. Environ Sci Technol 33:3892–3897. https://doi.org/10.1021/es990260k
Zavvar Mousavi H, Seyedi SR (2010) Kinetic and equilibrium studies on the removal of pb (ii) from aqueous solution using nettle ash. J Chil Chem Soc 55:307–311. https://doi.org/10.4067/S0717-97072010000300006
Naushad M, Alothman ZA, Awual MR, Alam MM, Eldesoky GE, Adsorption kinetics, isotherms, and thermodynamic studies for the adsorption of Pb2+ and Hg2+ metal ions from aqueous medium using Ti(IV) iodovanadate cation exchanger. Ionics 2237:224–221. https://doi.org/10.1007/s11581-015-1401-7)
Sharma P, Jindal R, Maiti M, Jana AK (2016) Novel organic–inorganic composite material as a cation exchanger from a triterpenoidal system of dammar gum: synthesis, characterization and application. Iran Polym J. https://doi.org/10.1007/s13726-016-0456-2
Pathania D, Gupta D, Al-Muhtaseb AH et al (2016) Photocatalytic degradation of highly toxic dyes using chitosan-g-poly(acrylamide)/ZnS in presence of solar irradiation. J Photochem Photobiol A Chem 329:61–68. https://doi.org/10.1016/j.jphotochem.2016.06.019
Naushad M, Mittal A, Rathore M, Gupta V (2015) Ion-exchange kinetic studies for Cd(II), Co(II), Cu(II), and Pb(II) metal ions over a composite cation exchanger. Desalin Water Treat 54:2883–2890. https://doi.org/10.1080/19443994.2014.904823
Naushad M, AL-Othman ZA, Islam M (2013) Adsorption of cadmium ion using a new composite cation-exchanger polyaniline Sn(IV) silicate: kinetics, thermodynamic and isotherm studies. Int J Environ Sci Technol 10:567–578. https://doi.org/10.1007/s13762-013-0189-0
Çay S, Uyanik A, Özaşik A (2004) Single and binary component adsorption of copper(II) andcadmium(II) from aqueous solutions using tea-industry waste. Sep Purif Technol 38:273–280. https://doi.org/10.1016/j.seppur.2003.12.003
Naushad M, ALOthman ZA, Javadian H (2015) Removal of Pb(II) from aqueous solution using ethylene diamine tetra acetic acid-Zr(IV) iodate composite cation exchanger: Kinetics, isotherms and thermodynamic studies. J Ind Eng Chem 25:35–41. https://doi.org/10.1016/j.jiec.2014.10.010
Bushra R, Naushad M, Adnan R et al (2015) Polyaniline supported nanocomposite cation exchanger: synthesis, characterization and applications for the efficient removal of Pb2+ ion from aqueous medium. J Ind Eng Chem 21:1112–1118. https://doi.org/10.1016/j.jiec.2014.05.022
Siddiqi ZM, Pathania D (2003) Studies on titanium(IV) tungstosilicate and titanium(IV) tungstophosphate. II. Separation and estimation of heavy metals from aquatic environments. Acta Chromatogr 172–184
Pathania D, Thakur M, Mishra AK (2017) Alginate–Zr (IV) phosphate nanocomposite ion exchanger: binary separation of heavy metals, photocatalysis and antimicrobial activity. Elsevier, Oxford
Kullberg L (1984) Edited by A. Clearfield (Texas ASM University). CRC Press, Inc, Raton B, Fl. 1982. 304 pp. $ 84.50. Solvent Extr Ion Exch 2:121–122. https://doi.org/10.1080/07366298408918440
Naushad M (2014) Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2 + from aqueous medium. Chem Eng J 235:100–108. https://doi.org/10.1016/j.cej.2013.09.013
Varshney KG, Khan AA, Siddiqui MS (1989) Synthesis, ion exchange behaviour and characterization of chromium(III) arsenosilicate cation exchanger. Colloids Surf 36:405–416. https://doi.org/10.1016/0166-6622(89)80255-0
New AA, Exchanger C, Varshney KG et al (1998) Synthesis and characterization of zirconium. 7353–7358
Jindal R, Sharma R, Maiti M, Kaur H (2016) In air synthesis of psyllium based organo- inorganic hybrid ion exchanger, its characterization and studies. 1:22–29
Narayana S, Graduate P (2006) Synthetic inorganic ion exchangers Reetha Nanu Cheruvalath “Studies on some ion exchangers ”
Saruchi KV, Kaith BS, Jindal R (2016) Synthesis of hybrid ion exchanger for rhodamine B dye removal: equilibrium, kinetic and thermodynamic studies. Ind Eng Chem Res 55:10492–10499. https://doi.org/10.1021/acs.iecr.6b01690
Pathania D, Sharma G, Thakur R (2015) Pectin @ zirconium (IV) silicophosphate nanocomposite ion exchanger: photo catalysis, heavy metal separation and antibacterial activity. Chem Eng J 267:235–244. https://doi.org/10.1016/j.cej.2015.01.004
Pathania D, Gupta D, Agarwal S et al (2016) Fabrication of chitosan-g-poly(acrylamide)/CuS nanocomposite for controlled drug delivery and antibacterial activity. Mater Sci Eng C 64:428–435. https://doi.org/10.1016/j.msec.2016.03.065
Kaith BS, Sharma J, Kaur T et al (2016) Microwave-assisted green synthesis of hybrid nanocomposite: removal of Malachite green from waste water. Iran Polym J (English Ed) 25:787–797. https://doi.org/10.1007/s13726-016-0467-z
Rana P, Mohan N, Rajagopal C (2004) Electrochemical removal of chromium from wastewater by using carbon aerogel electrodes. Water Res 38:2811–2820. https://doi.org/10.1016/j.watres.2004.02.029
Mobasherpour I, Salahi E, Pazouki M (2011) Removal of divalent cadmium cations by means of synthetic nano crystallite hydroxyapatite. Desalination 266:142–148. https://doi.org/10.1016/j.desal.2010.08.016
Ma Y, Zheng YM, Chen JP (2011) A zirconium based nanoparticle for significantly enhanced adsorption of arsenate: synthesis, characterization and performance. J Colloid Interface Sci 354:785–792. https://doi.org/10.1016/j.jcis.2010.10.041
Sharma G, Pathania D, Naushad M (2015) Preparation, characterization, and ion exchange behavior of nanocomposite polyaniline zirconium(IV) selenotungstophosphate for the separation of toxic metal ions. Ionics 21:1045–1055. https://doi.org/10.1007/s11581-014-1269-y
Peng Z, Peng Z, Shen Y (2011) Fabrication and properties of gelatin/chitosan composite hydrogel. Polym Plast Technol Eng 50:1160–1164. https://doi.org/10.1080/03602559.2011.574670
Mao JS, Zhao LG, Yin YJ, Yao K, De (2003) Structure and properties of bilayer chitosan–gelatin scaffolds. Biomaterials 24:1067–1074. https://doi.org/10.1016/S0142-9612(02)00442-8
Albadarin AB, Collins MN, Naushad M et al (2017) Activated lignin–chitosan extruded blends for efficient adsorption of methylene blue. Chem Eng J 307:264–272. https://doi.org/10.1016/j.cej.2016.08.089
Sharma G, Naushad M, Al-Muhtaseb AH et al (2017) Fabrication and characterization of chitosan-crosslinked-poly(alginic acid) nanohydrogel for adsorptive removal of Cr(VI) metal ion from aqueous medium. Int J Biol Macromol 95:484–493. https://doi.org/10.1016/j.ijbiomac.2016.11.072
Kumar A, Guo C, Sharma G et al (2016) Magnetically recoverable ZrO2/Fe3O4/chitosan nanomaterials for enhanced sunlight driven photoreduction of carcinogenic Cr(VI) and dechlorination and mineralization of 4-chlorophenol from simulated waste water. RSC Adv 6:13251–13263. https://doi.org/10.1039/c5ra23372k
Muhmood T, Xia M, Lei W, Wang F (2018) Under vacuum synthesis of type-I heterojunction between red phosphorus and graphene like carbon nitride with enhanced catalytic, electrochemical and charge separation ability for photodegradation of an acute toxicity category-III compound. Appl Catal B Environ 238:568–575. https://doi.org/10.1016/j.apcatb.2018.07.029
Therdthai N, Zhou W (2009) Characterization of microwave vacuum drying and hot air drying of mint leaves (Mentha cordifolia Opiz ex Fresen). J Food Eng 91:482–489. https://doi.org/10.1016/j.jfoodeng.2008.09.031
Deng H, Lu J, Li G et al (2011) Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem Eng J 172:326–334. https://doi.org/10.1016/j.cej.2011.06.013
Wang L, Zhang J, Wang A (2008) Removal of methylene blue from aqueous solution using chitosan-g-poly(acrylic acid)/montmorillonite superadsorbent nanocomposite. Colloids Surf A Physicochem Eng Asp 322:47–53. https://doi.org/10.1016/j.colsurfa.2008.02.019
Mi FL (2005) Synthesis and characterization of a novel chitosan-gelatin bioconjugate with fluorescence emission. Biomacromol 6:975–987. https://doi.org/10.1021/bm049335p
Sharma G, Kumar A, Pathania D, Sillanpa M (2016) Journal of Industrial and Engineering Chemistry Polyacrylamide @ Zr (IV) vanadophosphate nanocomposite: Ion exchange properties, antibacterial activity, and photocatalytic behavior. J Ind Eng Chem 33:201–208. https://doi.org/10.1016/j.jiec.2015.10.011
Siddiqi ZM, Pathania D (2003) Titanium(IV) tungstosilicate and titanium(IV) tungstophosphate: two new inorganic ion exchangers. J Chromatogr A 987:147–158
Kaith BS, Jindal R, Sharma R (2015) Synthesis of a Gum rosin alcohol-poly(acrylamide) based adsorbent and its application in removal of malachite green dye from waste water. RSC Adv 5:43092–43104. https://doi.org/10.1039/C5RA04256A
Fain SC, Sorensen L, Vilches OE (2011) Electron diffraction. 1–8
Naushad M, Ahamad T, Al-Maswari BM et al (2017) Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem Eng J 330:1351–1360. https://doi.org/10.1016/j.cej.2017.08.079
Alqadami AA, Naushad M, Alothman ZA, Ghfar AA (2017) Novel metal-organic framework (MOF) based composite material for the sequestration of U(VI) and Th(IV) metal ions from aqueous environment. ACS Appl Mater Interfaces 9:36026–36037. https://doi.org/10.1021/acsami.7b10768
Ovchinnikov O, Chernykh S, Smirnov MS et al (2007) Analysis of interaction between the organic dye methylene blue and the surface of AgCl (I) microcrystals. J Appl Spectrosc 74:731–737
Vargas AMM, Cazetta AL, Kunita MH et al (2011) Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem Eng J 168:722–730. https://doi.org/10.1016/j.cej.2011.01.067
Su F, Lu C, Hu S (2010) Adsorption of benzene, toluene, ethylbenzene and p-xylene by NaOCl-oxidized carbon nanotubes. Colloids Surf A Physicochem Eng Asp 353:83–91. https://doi.org/10.1016/j.colsurfa.2009.10.025
Fu Y, Huang Y, Hu J (2018) Preparation of chitosan/MCM-41-PAA nanocomposites and the adsorption behaviour of Hg (II) ions. R Soc Chem 171927
Kaur S, Jindal R, Kaur Bhatia J (2018) Synthesis and RSM–CCD optimization of microwave-induced green interpenetrating network hydrogel adsorbent based on gum copal for selective removal of malachite green from waste water. Polym Eng Sci 1–11. https://doi.org/10.1002/pen.24851
Das S, Subuddhi U (2013) Cyclodextrin mediated controlled release of naproxen from pH-sensitive chitosan/poly(vinyl alcohol) hydrogels for colon targeted delivery. Ind Eng Chem Res 52:14192–14200. https://doi.org/10.1021/ie402121f
Jindal R, Sharma R, Maiti M, Sharma A (2017) Synthesis and characterization of novel reduced gum rosin—acrylamide copolymer based bimetallic nanogel and their investigation for antimicrobial activity. Polym Bull 74:24–30
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124. https://doi.org/10.1016/S1385-8947(98)00076-X
Gupta VK, Agarwal S, Pathania D et al (2013) Use of pectin-thorium (IV) tungstomolybdate nanocomposite for photocatalytic degradation of methylene blue. Carbohydr Polym 96:277–283. https://doi.org/10.1016/j.carbpol.2013.03.073
Daneshvar E, Vazirzadeh A, Niazi A et al (2017) Desorption of Methylene blue dye from brown macroalga: effects of operating parameters, isotherm study and kinetic modeling. J Clean Prod 152:443–453. https://doi.org/10.1016/j.jclepro.2017.03.119
Khan MA, ALOthman ZA, Naushad M et al (2015) Adsorption of methylene blue on strongly basic anion exchange resin (Zerolit DMF): kinetic, isotherm, and thermodynamic studies. Desalin Water Treat 53:515–523. https://doi.org/10.1080/19443994.2013.838527
Naushad M, Ali Khan M, Abdullah Alothman Z et al (2016) Adsorption of methylene blue on chemically modified pine nut shells in single and binary systems: isotherms, kinetics, and thermodynamic studies. Desalin Water Treat 57:15848–15861. https://doi.org/10.1080/19443994.2015.1074121
Alqadami AA, Naushad M, Alothman ZA, Ahamad T (2018) Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: kinetics, isotherm and mechanism. J Environ Manag 223:29–36. https://doi.org/10.1016/j.jenvman.2018.05.090
Acknowledgements
One of the authors is highly grateful to MHRD for providing financial assistance to carry out research. The author is also thankful to Instrumentation Centre, IIT Roorkee, Materials research centre, MNIT Jaipur, SAIF facility, Punjab University, Chandigarh for different characterization of samples and DST-FIST for providing financial assistance for the procurement of equipment like FTIR and UV–Visible spectrophotometer used in the characterization of the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Rights and permissions
About this article
Cite this article
Kaur, K., Jindal, R. & Tanwar, R. Chitosan–Gelatin @ Tin (IV) Tungstatophosphate Nanocomposite Ion Exchanger: Synthesis, Characterization and Applications in Environmental Remediation. J Polym Environ 27, 19–36 (2019). https://doi.org/10.1007/s10924-018-1321-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1321-5