Abstract
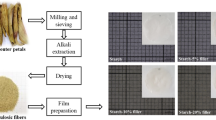
The potential of lignocellulosic fibers obtained by dry grinding of pinhão coat as fillers in starch filmogenic solutions for packaging applications was evaluated in this work. To improve the incorporation of this waste into the starch solutions different physical and chemical treatments were conducted. Thereafter, morphology, chemical structure, crystallinity and water absorption of the pinhão coat powders were determined. The composites were also characterized regarding their morphology, chemical structure, crystallinity, mechanical properties, water vapor permeability and hydrophilicity. Poor fiber/matrix adhesion and high water absorption of the fibers were evidenced. Consequently, water vapor permeability of composites was increased by incorporating the fibers. Moreover, mechanical properties were improved and the morphological results were used to support the water absorption differences among the powders. Regarding the food packaging applications, starch/pinhão coat composites appeared as promising materials to reach the requirements of respiring food products.




Similar content being viewed by others
References
Bogoeva-Gaceva G, Avella M, Malinconico M, Buzarovskja A, Grozdanov A, Gentile G, Errico ME (2007) Natural fiber eco-composites. Polym Compos 16:101–113. https://doi.org/10.1002/pc.20270
Dufresne A, Dupeyre D, Paillet M (2002) Lignocellulosic flour-reinforced poly(hydroxybutyrate-co-valerate) composites. J Appl Polym Sci 87:1302–1315. https://doi.org/10.1002/app.11546
Wong S, Shanks R, Hodzic A (2004) Interfacial improvements in poly(3-hydroxybutyrate)-flax fibre composites with hydrogen bonding additives. Compos Sci Technol 64:1321–1330. https://doi.org/10.1016/j.compscitech.2003.10.012
Bledzki AK, Mamun AA, Volk J (2010) Barley husk and coconut shell reinforced polypropylene composites: the effect of fibre physical, chemical and surface properties. Compos Sci Technol 70:840–846. https://doi.org/10.1016/j.compscitech.2010.01.022
Ahankari SS, Mohanty AK, Misra M (2011) Mechanical behaviour of agro-residue reinforced poly(3-hydroxybutyrate-co-3-hydroxyvalerate), (PHBV) green composites: a comparison with traditional polypropylene composites. Compos Sci Technol 71:653–657. https://doi.org/10.1016/j.compscitech.2011.01.007
Cunha M, Berthet M-A, Pereira R, Covas JA, Vicente AA, Hilliou L (2015) Development of polyhydroxyalkanoate/beer spent grain fibers composites for film blowing applications. Polym Compos 36:1859–1865. https://doi.org/10.1002/pc.23093
Berthet MA, Angellier-Coussy H, Machado D et al (2015) Exploring the potentialities of using lignocellulosic fibres derived from three food by-products as constituents of biocomposites for food packaging. Ind Crops Prod 69:110–122. https://doi.org/10.1016/j.indcrop.2015.01.028
Cordenunsi BR, De Menezes EW, Genovese MI et al (2004) Chemical composition and glycemic index of Brazilian pine (Araucaria angustifolia) seeds. J Agric Food Chem 52:3412–3416. https://doi.org/10.1021/jf034814l
Lima EC, Royer B, Vaghetti JCP et al (2007) Adsorption of Cu(II) on Araucaria angustifolia wastes: determination of the optimal conditions by statistic design of experiments. J Hazard Mater 140:211–220. https://doi.org/10.1016/j.jhazmat.2006.06.073
Brasil JL, Ev RR, Milcharek CD et al (2006) Statistical design of experiments as a tool for optimizing the batch conditions to Cr(VI) biosorption on Araucaria angustifolia wastes. J Hazard Mater 133:143–153. https://doi.org/10.1016/j.jhazmat.2005.10.002
EMBRAPA (2015) Empresa Brasileira de Pesquisa Agropecuária. https://www.embrapa.br/busca-de-noticias/-/noticia/1819540/cem-maneiras-de-preparar-o-pinhao. Accessed 6 June 2017
Ayrilmis N, Jarusombuti S, Fueangvivat V, Bauchongkol P (2011) Effect of thermal-treatment of wood fibres on properties of flat-pressed wood plastic composites. Polym Degrad Stab 96:818–822. https://doi.org/10.1016/j.polymdegradstab.2011.02.005
Singh B, Gupta M, Verma A (1996) Influence of fiber surface treatment on the properties of sisal-polyester composites. Polym Compos 17:910–918. https://doi.org/10.1002/pc.10684
Miranda CS, Fiuza RP, Carvalho RF, José NM (2014) Effect of surface treatment on properties of bagasse piassava fiber Attalea funifera Martius. Quim Nova 38:161–165. https://doi.org/10.5935/0100-4042.20140303
d’Almeida ALFS., Calado V, Barreto DW, d’Almeida JRM (2005) Acetilação da fibra de bucha (Luffa cylindrica). Polímeros 15:59–62. https://doi.org/10.1590/S0104-14282005000100013
Lü J, Zhong J, Wei C (2006) Studies on the properties of sisal fibre/phenol formaldehyde resin in-situ composites. Res J Text Appar 10:51–58. https://doi.org/10.1108/RJTA-10-03-2006-B007
AOAC (2005) Official methods of analysis AOAC, 18th edn. AOAC Int., Arlington
Schirmer-Michel ÂC, Flôres SH, Hertz PF et al (2008) Production of ethanol from soybean hull hydrolysate by osmotolerant Candida guilliermondii NRRL Y-2075. Bioresour Technol 99:2898–2904. https://doi.org/10.1016/j.biortech.2007.06.042
Ramadevi P, Sampathkumar D, Srinivasa CV, Bennehalli B (2012) Effect of alkali treatment on water absorption of single cellulosic abaca fiber. BioResources 7:3515–3524. https://doi.org/10.15376/biores.7.3.3515-3524
Phan TD, Debeaufort F, Luu D, Voilley A (2005) Functional properties of edible agar-based and starch-based films for food quality preservation. J Agric Food Chem 53:973–981. https://doi.org/10.1021/jf040309s
ASTM International (2002) Standard test methods for water vapor transmission of materials 1. ASTM 14:1–10. https://doi.org/10.1520/E0096
ASTM International (2012) ASTM D882: standard test method for tensile properties of thin plastic sheeting. ASTM Stand. https://doi.org/10.1520/D0882-12.2
CETEC (2006) Fundação Centro Tecnológico de Minas Gerais. Pinhão manso. Belo Horizonte, http://www.pinhaomanso.com.br/propiedades.html
Mielenz JR, Bardsley JS, Wyman CE (2009) Fermentation of soybean hulls to ethanol while preserving protein value. Bioresour Technol 100:3532–3539. https://doi.org/10.1016/j.biortech.2009.02.044
Mussatto SI, Dragone G, Roberto IC (2006) Brewers’ spent grain: generation, characteristics and potential applications. J Cereal Sci 43:1–14. https://doi.org/10.1016/j.jcs.2005.06.001
Laser M, Schulman D, Allen SG et al (2002) A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour Technol 81:33–44. https://doi.org/10.1016/S0960-8524(01)00103-1
Mello LRPF., Mali S (2014) Use of malt bagasse to produce biodegradable baked foams made from cassava starch. Ind Crops Prod 55:187–193. https://doi.org/10.1016/j.indcrop.2014.02.015
Venkateshappa SC, Bennehalli B, Kenchappa MG, Ranganagowda RPG (2010) Flexural behaviour of areca fibers composites. BioResources 5:1846–1858
Bennehalli B, Sampathkumar D, Punyamurth R, Venkateshappa SC (2012) Effect of esterification on moisture absorption of single areca fiber. Int J Agric Sci 4:227–229. https://doi.org/10.9735/0975-3710.4.4.227-229
Wu M, Wang LJ, Li D et al (2013) Effect of flaxseed meal on the dynamic mechanical properties of starch-based films. J Food Eng 118:365–370. https://doi.org/10.1016/j.jfoodeng.2013.04.017
Berthet MA, Angellier-Coussy H, Chea V et al (2015) Sustainable food packaging: valorising wheat straw fibres for tuning PHBV-based composites properties. Compos A 72:139–147. https://doi.org/10.1016/j.compositesa.2015.02.006
Belibi PC, Daou TJ, Ndjaka JMB et al (2014) A comparative study of some properties of cassava and tree cassava starch films. Phys Procedia 55:220–226. https://doi.org/10.1016/j.phpro.2014.07.032
Maran JP, Sivakumar V, Sridhar R, Thirugnanasambandham K (2013) Development of model for barrier and optical properties of tapioca starch based edible films. Carbohydr Polym 92:1335–1347. https://doi.org/10.1016/j.carbpol.2012.09.069
Chang YP, Cheah PB, Seow CC (2000) Plasticizing-antiplasticizing effects of water on physical properties of tapioca starch films in the glassy state. J Food Sci 65:445–451. https://doi.org/10.1111/j.1750-3841.2000.00445.pp.x
Wang J, Jin Z, Yuan X (2007) Preparation of resistant starch from starch-guar gum extrudates and their properties. Food Chem 101:20–25. https://doi.org/10.1016/j.foodchem.2006.01.005
Tongdeesoontorn W, Mauer LJ, Wongruong S et al (2011) Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem Cent J 5:6. https://doi.org/10.1186/1752-153X-5-6
Mehyar GF, Han JH (2004) Physical and mechanical properties of high-amylose rice and pea starch films as affected by relative humidity and plasticizer. J Food Sci 69:449–454
Melgosa M, Pérez MM, Yebra A, Huertas R, Hita E (2001) Algunas reflexiones y recientes recomendaciones internacionales sobre evaluación de diferencias de color. Opt Pura Apl 34:1–10
Lee HS, Coates GA (2003) Effect of thermal pasteurization on Valencia orange juice color and pigments. LWT 36:153–156. https://doi.org/10.1016/S0023-6438(02)00087-7
Ludueña L, Vázquez A, Alvarez V (2012) Effect of lignocellulosic filler type and content on the behavior of polycaprolactone based eco-composites for packaging applications. Carbohydr Polym 87:411–421. https://doi.org/10.1016/j.carbpol.2011.07.064
Müller CMO, Laurindo JB, Yamashita F (2009) Effect of cellulose fibers on the crystallinity and mechanical properties of starch-based films at different relative humidity values. Carbohydr Polym 77:293–299. https://doi.org/10.1016/j.carbpol.2008.12.030
Aila-Suárez S, Palma-Rodríguez HM, Rodríguez-Hernández AI et al (2013) Characterization of films made with chayote tuber and potato starches blending with cellulose nanoparticles. Carbohydr Polym 98:102–107. https://doi.org/10.1016/j.carbpol.2013.05.022
Santacruz S, Rivadeneira C, Castro M (2015) Edible films based on starch and chitosan. Effect of starch source andconcentration, plasticizer, surfactant’s hydrophobic tail andmechanical treatment. Food Hydrocoll 49:89–94. https://doi.org/10.1016/j.foodhyd.2015.03.019
McHugh TH, Avenabustillos R, Krochta JM (1993) Hydrophilic edible films: modified procedure for water-vapor permeability and explanation of thickness effects. J Food Sci 58:899–903. https://doi.org/10.1111/j.1365-2621.1993.tb09387.x
Almrhag O, George P, Bannikova A et al (2012) Networks of polysaccharides with hydrophilic and hydrophobic characteristics in the presence of co-solute. Int J Biol Macromol 51:138–145. https://doi.org/10.1016/j.ijbiomac.2012.04.015
Silverstein RM, Bassler GC, Morrill TC (1981) Spectrometric identification of organic compounds, 4th edn. Wiley, Hoboken. (ISBN 10: 0471029904/ISBN 13: 9780471029908$4)
Kalia S, Kaith BS, Kaur I (2009) Pretreatments of natural fibers and their application as reinforcing material in polymer composites—a review. Polym Eng Sci 1253–1272. https://doi.org/10.1002/pen
Slavutsky AM, Bertuzzi MA (2016) Improvement of water barrier properties of starch films by lipid nanolamination. Food Packag Shelf Life 7:41–46. https://doi.org/10.1016/j.fpsl.2016.01.004
Nascimento TA, Calado V, Carvalho CWP (2012) Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res Int 49:588–595. https://doi.org/10.1016/j.foodres.2012.07.051
Acknowledgements
The authors acknowledge the financial support received from CAPES (Coordenadoria de Aperfeiçoamento de Pessoal para o Ensino Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul). In particular, the authors thank the CAPES CSF-PVE’s Project, process number: 88881.068177/2014-01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spada, J.C., Luchese, C.L. & Tessaro, I.C. Potential of pinhão Coat as Constituents of Starch Based Films Using Modification Techniques. J Polym Environ 26, 2686–2697 (2018). https://doi.org/10.1007/s10924-017-1158-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1158-3