Abstract
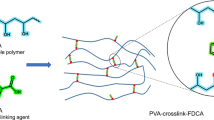
In the presented work, the effect of crosslinker geometry on the properties of PVA is reported. The aliphatic (suberic) and aromatic (terephthalic) dicarboxylic acids are used as crosslinker molecules. On the basis of tensile test and thermal properties, it is observed that crosslinking of PVA by suberic acid is more effective than terephthalic acid. The maximum strength measured in crosslinked samples is 32.5 MPa for suberic acid crosslinked PVA which is higher than that of neat PVA (22.6 MPa). Swelling study shows that 8 h crosslinked terephthalic acid (35% w/w) samples have a minimum of 5.4% of water uptake compared to neat PVA, which dissolves readily in water. DTGA shows that the decomposition temperature of crosslinked PVA is 345 °C while neat PVA has a decomposition temperature of 315 °C. FTIR spectroscopy confirms the formation of crosslink ester bond in crosslinked PVA. The crosslinked samples kept for bio-degradation show maximum degradation in terephthalic acid (15% w/w) crosslinked PVA.










Similar content being viewed by others
References
Horii F, Masuda K (1998) Chap. 19 Hydrogen-bonded polymers. In: Isao A, Tetsou A (eds) Studies in physical and theoretical chemistry, vol 84. Elsevier, Amsterdam, pp 713–736
Earl PA (1965) Polyvinyl acetate and polyvinyl alcohol adhesives. US3213051 A (US patent)
Zaisheng Cai, Yiping Qiu, Chuyang Zhang, Hwang Y-J, Mccord M (2003) Effect of atmospheric plasma treatment on desizing of PVA on cotton. Text Res J 73(8):670–674
Rosenblatt KM, Bunjes H (2009) Poly(vinyl alcohol) as emulsifier stabilizes solid triglyceride drug carrier nanoparticles in the α-modification. Mol Pharm 6(1):105–120
Bolto B, Tran T, Hoang M, Xie Z (2009) Crosslinked poly(vinyl alcohol) membranes. Prog Polym Sci 34(9):969–981
Oshima K, Iwasaki S (1990) Pneumatic radial tires with a folded belt and high strength vinylon fiber cords. US4971127 A (US patent)
Mohanty S, Larsen LB, Trifol J, Szabo P, Burri HVR, Canali C, Dufva M, Emnéus J, Wolff A (2015) Fabrication of scalable and structured tissue engineering scaffolds using water dissolvable sacrificial 3D printed moulds. Mater Sci Eng C 55:569–578
Heydari M, Moheb A, Ghiaci M, Masoomi M (2013) Effect of crosslinking time on the thermal and mechanical properties and pervaporation performance of poly(vinyl alcohol) membrane crosslinked with fumaric acid used for dehydration of isopropanol. J Appl Polym Sci 128(3):1640–1651
Işıklan N, Şanlı O (2005) Separation characteristics of acetic acid–water mixtures by pervaporation using poly(vinyl alcohol) membranes modified with malic acid. Chem Eng Process 44(9):1019–1027
Caro SV, Sung CSP, Merrill EW (1976) Reaction of hexamethylene diisocyanate with polyvinyl alcohol films for biomedical applications. J Appl Polym Sci 20(12):3241–3246
Arranz F, Sánchez-Chaves M, Martínez R (1987) Reaction of poly(vinyl alcohol) with n-butyl isocyanate. Chemical hydrolysis of the resulting polymers. Die Angewandte Makromolekulare Chemie 152(1):79–91
Arranz F, Bejarano EM, Sanchez-Chaves M (1994) Poly(vinyl alcohol) functionalized by chloroacetate groups. Coupling of bioactive carboxylic acids. Macromol Chem Phys 195(12):3789–3798
Ichimura K, Watanabe S (1982) Preparation and characteristics of photocrosslinkable poly(vinyl alcohol). J Polym Sci 20(6):1419–1432
Ichimura K (1982) Preparation of water-soluble photoresist derived from poly(vinyl alcohol). J Polym Sci 20(6):1411–1417
Sonker AK, Tiwari N, Nagarale RK, Verma V (2016) Synergistic effect of cellulose nanowhiskers reinforcement and dicarboxylic acids crosslinking towards polyvinyl alcohol properties. J Polym Sci 54(16):2515–2525
Suzuki T, Ichihara Y, Yamada M, Tonomura K (1973) Some characteristics of Pseudomonas 0–3 which utilizes polyvinyl alcohol. Agric Biol Chem 37(4):747–756
Chiellini E, Corti A, D’Antone S, Solaro R (2003) Biodegradation of poly (vinyl alcohol) based materials. Prog Polym Sci 28(6):963–1014
Figueiredo KCS, Alves TLM, Borges CP (2009) Poly(vinyl alcohol) films crosslinked by glutaraldehyde under mild conditions. J Appl Polym Sci 111(6):3074–3080
Campos E, Coimbra P, Gil MH (2013) An improved method for preparing glutaraldehyde crosslinked chitosan–poly(vinyl alcohol) microparticles. Polym Bull 70(2):549–561
Beydaghi H, Javanbakht M, Badiei A (2014) Crosslinked poly(vinyl alcohol)/sulfonated nanoporous silica hybrid membranes for proton exchange membrane fuel cell. J Nanostruct Chem 4(2):1–9
Peng F, Hu C, Jiang Z (2007) Novel ploy(vinyl alcohol)/carbon nanotube hybrid membranes for pervaporation separation of benzene/cyclohexane mixtures. J Membr Sci 297(1–2):236–242
Namboodiri VV, Ponangi R, Vane LM (2006) A novel hydrophilic polymer membrane for the dehydration of organic solvents. Eur Polym J 42(12):3390–3393
Wang X, Fang D, Yoon K, Hsiao BS, Chu B (2006) High performance ultrafiltration composite membranes based on poly(vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly(vinyl alcohol) scaffold. J Membr Sci 278(1–2):261–268
Zhang Y, Li H, Li H, Li R, Xiao C (2006) Preparation and characterization of modified polyvinyl alcohol ultrafiltration membranes. Desalination 192(1–3):214–223
Liu Q-L, Li Q-B (2002) Membrane of PVA coated on porous catalytic ceramic disks supported H3PW12O40. J Membr Sci 202(1–2):89–95
Huang RYM, Rhim JW (1993) Modification of poly(vinyl alcohol) using maleic acid and its application to the separation of acetic acid-water mixtures by the pervaporation technique. Polym Int 30(1):129–135
Rhim J-W, Park HB, Lee C-S, Jun J-H, Kim DS, Lee YM (2004) Crosslinked poly(vinyl alcohol) membranes containing sulfonic acid group: proton and methanol transport through membranes. J Membr Sci 238(1–2):143–151
Dlamini DS, Wang J, Mishra AK, Mamba BB, Hoek EMV (2013) Effect of crosslinking agent chemistry and coating conditions on physical, chemical, and separation properties of PVA-Psf composite membranes. Sep Sci Technol 49(1):22–29
Jian S, Xiao Ming S (1987) Crosslinked PVA-PS thin-film composite membrane for reverse osmosis. Desalination 62(0):395–403
Krumova M, López D, Benavente R, Mijangos C, Pereña JM (2000) Effect of crosslinking on the mechanical and thermal properties of poly(vinyl alcohol). Polymer 41(26):9265–9272
Miyazaki T, Takeda Y, Akane S, Itou T, Hoshiko A, En K (2010) Role of boric acid for a poly (vinyl alcohol) film as a crosslinking agent: melting behaviors of the films with boric acid. Polymer 51(23):5539–5549
Wan YZ, Luo H, He F, Liang H, Huang Y, Li XL (2009) Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos Sci Technol 69(7–8):1212–1217
Miao T, Miller EJ, McKenzie C, Oldinski RA (2015) Physically crosslinked polyvinyl alcohol and gelatin interpenetrating polymer network theta-gels for cartilage regeneration. J Mater Chem B 3(48):9242–9249
Cutiongco MF, Choo RK, Shen NJ, Chua BM, Sju E, Choo AW, Le Visage C, Yim EK (2015) Composite scaffold of poly(vinyl alcohol) and interfacial polyelectrolyte complexation fibers for controlled biomolecule delivery. Front Bioeng Biotechnol 3:3
Kim JH, Moon EJ, Kim CK (2003) Composite membranes prepared from poly(m-animostyrene-co-vinyl alcohol) copolymers for the reverse osmosis process. J Membr Sci 216(1–2):107–120
Blout ER, Karplus R (1948) The infrared spectrum of polyvinyl alcohol. J Am Chem Soc 70(2):862–864
Refojo MF (1965) Permeation of water through some hydrogels. J Appl Polym Sci 9(10):3417–3426
Yasuda H, Lamaze CE, Peterlin A (1971) Diffusive and hydraulic permeabilities of water in water-swollen polymer membranes. J Polym Sci Part A-2 9(6):1117–1131
Yasuda H, Lamaze CE (1971) Salt rejection by polymer membranes in reverse osmosis. I. Nonionic polymers. J Polym Sci Part A-2 9(9):1537–1551
Gohil JM, Bhattacharya A, Ray P (2006) Studies on the crosslinking of poly (vinyl alcohol). J Polym Res 13(2):161–169
Flory PJ, Rehner J (1943) Statistical mechanics of cross-linked polymer networks II. swelling. J Chem Phys 11(11):521–526
Yang M-H, Chu T-J (1993) The determination of interaction parameter χ1 for polyvinyl alcohol and water from the diffusion data. Polym Test 12(1):57–64
Ganji F, Vasheghani-Farahani S, Vasheghani-Farahani E (2010) Theoretical description of hydrogel swelling: a review. Iran Polym J 19(5):375–398
Mooney RCL (1941) An X-ray study of the structure of polyvinyl alcohol. J Am Chem Soc 63(10):2828–2832
Assender HE, Windle AH (1998) Crystallinity in poly(vinyl alcohol). 1. An X-ray diffraction study of atactic PVOH. Polymer 39(18):4295–4302
Ahmad J, Hägg M-B (2013) Preparation and characterization of polyvinyl acetate/zeolite 4 A mixed matrix membrane for gas separation. J Membr Sci 427:73–84
Ahmad J, Hågg MB (2013) Polyvinyl acetate/titanium dioxide nanocomposite membranes for gas separation. J Membr Sci 445:200–210
Bunn CW (1948) Crystal structure of polyvinyl alcohol. Nature 161:929–930
Post B (1971) X-ray diffraction methods in polymer science, Leroy E. Alexander, wiley-interscience, new york, 1970. xv + 582 pp. J Polym Sci Part B 9(8):635–636
Acknowledgements
RKN thanks the Department of Science & Technology (DST), Government of India, for Ramanujan Fellowship (SR/S2/RJN-18/2011) award. The work is funded by Science and Engineering Research Board (SERB) SR/S3/CE/038/2012 granted to VV.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sonker, A.K., Rathore, K., Nagarale, R.K. et al. Crosslinking of Polyvinyl Alcohol (PVA) and Effect of Crosslinker Shape (Aliphatic and Aromatic) Thereof. J Polym Environ 26, 1782–1794 (2018). https://doi.org/10.1007/s10924-017-1077-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1077-3