Abstract
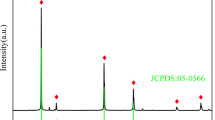
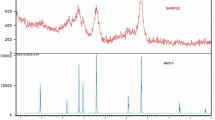
A series of nanaoscale aramid-based adsorbents were prepared by the functionalization of poly (p-phenylene terephthalamide) (PPTA) with different content of ethylenediamine (EDA). Their structures were characterized by field emission scanning electron microscopy, transmission electron microscopy, Fourier transform infrared spectroscopy, thermogravimetric analysis, and elemental analysis. Metal ions, including Hg2+, Pb2+, Ag+, Cu2+, Cd2+, and Ni2+ were chosen as the models to explore the binding behaviors of PPTA–ECH–EDA in aqueous medium. Results showed that PPTA–ECH–EDA exhibited higher adsorption capacity for Hg2+ due to their nanoscale structures. In particular, the adsorption rate was so high that equilibrium was achieved within 15 min for Hg2+. The adsorption of Hg2+ on PPTA–ECH–EDA followed the pseudo second-order model well. Langmuir and Freundlich models were employed to fit the isothermal adsorption, and the results revealed that Freundlich isotherm was a better model to predict the experimental data. The adsorption mechanism was revealed by X-ray photoelectron spectroscopy. It is preconceived that PPTA–ECH–EDA could be used as an effective adsorbent for fast removal of heavy ions from wastewater.















Similar content being viewed by others
References
Qu RJ, Sun XY, Sun CM, Zhang Y, Wang CH, Ji CN, Chen H (2012) Chem Eng J 182:458
Xi M, Li YL, Shang SY, Li DH, Yin YX, Dai XY (2008) Surf Coat Technol 202:6029
Hanv WH, Zhao TY, Wang XG (2015) Polymer 57:150
Park JM, Kim DS, Kim SR (2003) J Colloid Interface Sci 264:431
Every HA, Janssen GJM, Sitters EF, Mendes E, Picken SJ (2006) Power Sources 162:380
Muniz AC, Paredes JL, Alonso AM, Tascon JMD (2010) Polym Degrad Stab 95:702
Takayanagi M, Katayose T (1981) J Poly Sci Part A Polym Chem 19:1133
Wang C, Xiao CF, Huang QL (2015) J Membr Technol 474:132
Biggs BS, Frosch CJ, Erickson RH (1946) Ind Eng Chem 38:1016
Takayanagiv M, Goto K (1984) J Appl Polym Sci 29:2547
Takayanagi M, Goto K (1985) Polym Bull 13:35
Burch RR, Sweeny W, Schmidt HW, Kim YH (1990) Macromolecules 23:1065
O’Connor I, Hayden H, O’Connor S, Coleman JN, Gun’Ko YK (2009) J Phys Chem C 113:20184
Wang Y, Shi ZX, Yin J (2011) Polymer 52:3661
Fan J, Wang J, Shi ZX, Yu S, Yin J (2013) Mater Chem Phys 141:861
Qu RJ, Sun XY, Sun CM, Ji CN, Wang CH (2012) Polym Adv Technol 23:21
Suharso B (2010) Desalination 263:64
Naiya TK, Bhattacharya AK, Das SK (2009) J Hazard Mater 170:252
Tsou L, Sauer JA, Hara M (2000) Polymer 35:8103
Cai RQ, Peng T, Wang FD (2011) Appl Surf Sci 257:9562
Zhang Y, Qu RJ, Sun CM, Chen H (2009) J Hazard Mater 163:127
Ho YS, Mckay G (1999) Process Biochem 34:451
Wu Z, Joo H, Lee K (2005) Chem Eng J 112:227
Wang SB, Li HT (2005) J Hazard Mater B126:71
Ho YS (2006) Water Res 40:119
Ramesh A, Hasegawa H, Sugimoto W, Maki T (2008) Bioresour Technol 99:3801
Zhou ML, Martin G, Taha S (1996) Water Res 32:1109
Ji CN, Song SH, Wang CR (2010) Chem Eng J 165:573
Qu RJ, Zhang Y, Sun CM, Wang CH, Ji CN (2010) Chem Eng Data 55:1496
Ma F, Qu RJ, Sun CM, Wang CH, Ji CN (2009) J Hazard Mater 172:792
Volesky B, Holan ZR (1995) Biotechnol Prog 11:235
Ahmed MH, Byrne JA, Mclaughlin JAD (2013) Appl Surf Sci 273:507
Majumder S, Priyadarshini M, Subudhi U, Chainy GBN, Varma S (2009) Appl Surf Sci 256:438
Sun CM, Qu RJ, Ji CN, Wang Q, Wang CH (2006) Eur Polym J 42:188
Lim SF, Zheng YM, Zou SW, Chen JP (2008) Environ Sci Techno 42:2551
Gupta VK, Singh P, Rahman N (2004) J Colloid Interface Sci 2:398
Anirudhan TS, Jalajamony S, Sreekumari SS (2012) J Appl Clay Sci 65–66:67
Liu Y, Sun XM, Li BH (2010) Carbohydr Polym 81:335
Acknowledgments
The authors are grateful for the financial support by the National Natural Science Foundation of China (Grant Nos. 51373074, 51073075, 51302127, 51143006).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, C., Qu, R., Li, S. et al. Preparation, Characterization, and Rapid Adsorption of Hg2+ on Nanoscale Aramid-based Adsorbent. J Polym Environ 24, 206–220 (2016). https://doi.org/10.1007/s10924-016-0764-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0764-9