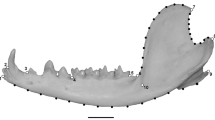
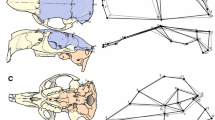
Abstract
Understanding the interplay between morphological integration and modularity is considered an important topic in the study of the evolution of the form of complex structures. The mandible is a complex structure that can be shaped by diverse factors such as ontogeny, ecology, and evolutionary history. In canids, this is particularly interesting because they have a large diversity in feeding behavior and hunting strategy. Here, we employed geometric morphometric techniques to evaluate the balance between integration and modularity in 1011 mandibles of a sample of extinct and extant canids. The results show that allometric scaling seems to have little influence in determining the mandibular shape of canids. Some divergence associated with ecology was observed, especially for highly specialized taxa (hypercarnivores and insectivores). Finally, macroevolutionary patterns were more integrated than intraspecific patterns, suggesting that correlational selection might play a strong role in the evolution of mandibular form and function. We found no evidence of an evolutionary line of least resistance in shaping mandible disparity.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adams DC (2014) A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst Biol 63:685–697
Adams DC (2016) Evaluating modularity in morphometric data: challenges with the RV coefficient and a new test measure. Methods Ecol Evol 7:565–572. https://doi.org/10.1111/2041-210X.12511
Adams DC, Felice RN (2014) Assessing trait covariation and morphological integration on phylogenies using evolutionary covariance matrices. PLoS One 9:e94335–e94338
Adams DC, Otárola-Castillo E (2013) Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399
Biknevicius AR, Ruff CB (1992) Use of biplanar radiographs for estimating cross-sectional geometric properties of mandibles. Anat Rec 232:157–163
Bookstein FL (1991) Morphometric Tools for Landmark Data. Geometry and Biology. Cambridge University Press, New York
Bubadué J de M, Cáceres N, Santos Carvalho R dos, Meloro C (2016) Ecogeographical variation in skull shape of South-American canids: abiotic or biotic processes? Evol Biol 43: 145–159
Cerny R, Lwigale P, Ericsson R, Meulemans D, Epperlein H. H, Bronner-Fraser M (2004) Developmental origins and evolution of jaws: new interpretation of “maxillary” and “mandibular.” Dev Biol 276:225–236
Cheverud JM (1996) Developmental integration and the evolution of pleiotropy. Am Zool 36:44–50
Christiansen P (2008) Evolution of skull and mandible shape in cats (Carnivora: Felidae). PLoS One 3:e2807
Clark HO (2005) Otocyon megalotis. Mammal Species 766:1–5
Conith AJ, Meagher MA, Dumont ER (2018) The influence of climatic variability on morphological integration, evolutionary rates, and disparity in the Carnivora. Am Nat 191:704–715
Curth S, Fischer MS, Kupczik K (2017) Patterns of integration in the canine skull: an inside view into the relationship of the skull modules of domestic dogs and wolves. Zoology 125:1–9
Echarri S, Prevosti FJ (2015) Differences in mandibular disparity between extant and extinct species of metatherian and placental carnivore clades. Lethaia 48:196–204
Emerson SB, Radinsky LB (1980) Functional analysis of sabretooth cranial morphology. Paleobiology 6:295–312
Esteve-Altava B (2017) In search of morphological modules: a systematic review. Biol Rev 92:1332–1347
Ewer RF (1998) The Carnivores. Cornell University Press, Ithaca
Figueirido B, Serrano-Alarcón FJ, Slater GJ, Palmqvist P (2010) Shape at the cross-roads: homoplasy and history in the evolution of the carnivoran skull towards herbivory. J Evol Biol 23:2579–2594
Garcia GRG, Hingst-Zaher E, Cerqueira R, Marroig G (2014) Quantitative genetics and modularity in cranial and mandibular morphology of Calomys expulsus. Evol Biol 41:619–636. doi: https://doi.org/10.1007/s11692-014-9293-4
Goodall CR (1991) Procrustes methods in the statistical analysis of shape. J Roy Stat Soc Ser B (Methodological) 53:285–339
Greaves WS (1982) A mechanical limitation on the position of the jaw muscles of mammals: the one-third rule. J Mammal 63:261–266
Greaves WS (1983) A functional analysis of carnassial biting. Biol J Linnean Soc 20:353–363
Hallgrímsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS (2009) Deciphering the palimpsest: studying the relationship between morphological integration and phenotypic covariation. Evol Biol 36:355–376
Hansen T (1997) Stabilizing selection and the comparative analysis of adaptation. Evolution 51:1341–1351
Hansen T, Houle D (2008) Measuring and comparing evolvability and constraint in multivariate characters. J Evol Biol 21:1201–1219
Harmon LJ, Schulte JA, Larson A, Losos JB (2003) Tempo and mode of evolutionary radiation in iguanian lizards. Science 301:961–964
Holliday JA, Steppan SJ (2004) Evolution of hypercarnivory: the effect of specialization on morphological and taxonomic diversity. Paleobiology 30:108–128
Klingenberg CP (2008) Morphological integration and developmental modularity. Annu Rev Ecol Evol Syst 39:115–132
Klingenberg CP (2009) Morphometric integration and modularity in configurations of landmarks: tools for evaluating a priori hypotheses. Evol Dev 11:405–421
Klingenberg CP, Leamy LJ, Cheverud JM (2004) Integration and modularity of quantitative trait locus effects on geometric shape in the mouse mandible. Genetics 166:1909–1921
La Croix S, Holekamp KE, Shivik JA, Lundrigan BL, Zelditch ML (2011a) Ontogenetic relationships between cranium and mandible in coyotes and hyenas. J Morphol 272:662–674
La Croix S, Zelditch ML, Shivik JA, Lundrigan BL, Holekamp KE (2011b) Ontogeny of feeding performance and biomechanics in coyotes. J Zool 285:301–315
Larouche O, Zelditch ML, Cloutier R (2018) Modularity promotes morphological divergence in ray-finned fishes. Sci Rep 8:7278
Machado FA, Hingst-Zaher E (2009) Investigating South American biogeographic history using patterns of skull shape variation on Cerdocyon thous (Mammalia: Canidae). Biol J Linnean Soc 98:77–84
Machado FA, Teta P (2020) Morphometric analysis of skull shape reveals unprecedented diversity of African Canidae. J Mammal
Machado FA, Zahn TMG, Marroig G (2018) Evolution of morphological integration in the skull of Carnivora (Mammalia): changes in Canidae lead to increased evolutionary potential of facial traits. Evolution 72:1399–1419
Marroig G, Cheverud JM (2010) Size as a line of least resistance II: direct selection on size or correlated response due to constraints? Evolution 64:1470–1488
Martinez PA, Pia MV, Bahechar IA, Molina WF, Bidau CJ, Montoya-Burgos JI (2018) The contribution of neutral evolution and adaptive processes in driving phenotypic divergence in a model mammalian species, the Andean fox Lycalopex culpaeus. J Biogeogr 3:595–512
Meloro C, Hudson A, Rook L (2014) Feeding habits of extant and fossil canids as determined by their skull geometry. J Zool 295:178–188
Meloro C, O’Higgins P (2011) Ecological adaptations of mandibular form in fissiped Carnivora. J Mammal Evol 18:185–200
Meloro C, Raia P, Carotenuto F, Cobb SN (2011) Phylogenetic signal, function and integration in the subunits of the carnivoran mandible. Evol Biol 38:465–475
Meloro C, Raia P, Piras P, Barbera C, O’Higgins P (2008) The shape of the mandibular corpus in large fissiped carnivores: allometry, function and phylogeny. Zool J Linn Soc 154:832–845
Mitteroecker P, Bookstein FL (2007) The conceptual and statistical relationship between modularity and morphological integration. Syst Zool 56:818
Mitteroecker P, Bookstein F (2008) The evolutionary role of modularity and integration in the hominoid cranium. Evolution 62:943–958
Muñoz NA, Cassini GH, Candela AM, Vizcaíno SF (2017) Ulnar articular surface 3-D landmarks and ecomorphology of small mammals: a case study of two early Miocene typotheres (Notoungulata) from Patagonia. Earth Env Sci Trans R Soc 106:315–323
Murrell DJ (2018) A global envelope test to detect non-random bursts of trait evolution. Methods Ecol Evol 9:1739–1748
Nowak RM (2005) Walker’s Carnivores of the World. John Hopkins University Press, London
Olson EC, Miller RL (1958) Morphological Integration. University of Chicago Press, Chicago
Oudot M, Neige P, Laffont R, Navarro N, Khaldi AY, Crônier C (2019) Functional integration for enrolment constrains evolutionary variation of phacopid trilobites despite developmental modularity. Palaeontology 4:393–317. https://doi.org/10.1111/pala.12428
Parr WC, Wilson LA, Wroe S, Colman NJ, Crowther MS, Letnic M (2016) Cranial shape and the modularity of hybridization in dingoes and dogs; hybridization does not spell the end for native morphology. Evol Biol 43:171–187.
Penrose F, Kemp GJ, Jeffery N (2016) Scaling and accommodation of jaw adductor muscles in Canidae. Anat Rec 299:951–966
Polly PD, Lawing AM, Fabre AC, Goswami A (2013) Phylogenetic principal components analysis and geometric morphometrics. Hystrix 24:33–41
Porto A, Shirai LT, Oliveira FB, Marroig G (2013) Size variation, growth strategies, and the evolution of modularity in the mammalian skull. Evolution 67:3305–3322. https://doi.org/10.1111/evo.12177
Prevosti FJ, Turazzini GF, Ercoli MD, Hingst-Zaher E (2011) Mandible shape in marsupial and placental carnivorous mammals: a morphological comparative study using geometric morphometrics. Zool J Linn Soc 164:836–855
Prevosti FJ, Segura V, Cassini GH (2013) Revision of the systematic status of Patagonian and Pampean gray foxes (Canidae: Lycalopex griseus and L. gymnocercus) using 3D geometric morphometrics. Mastozool Neotrop 20:289–300
R Core Team (2018) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Radinsky LB (1981a) Evolution of skull shape in carnivores 1. Representative modern carnivores. Biol J Linnean Soc 15:369–388
Radinsky LB (1981b) Evolution of skull shape in carnivores 2. Additional modern carnivores. Biol J Linnean Soc 16:337–355
Radinsky LB (1982) Evolution of skull shape in carnivores. 3. The origin and early radiation of the modern carnivore families. Paleobiology 8:177–195
Rayner JM (1985) Linear relations in biomechanics: the statistics of scaling functions. J Zool 206:415–439
Revell LJ (2009) Size-correction and principal components for interspecific comparative studies. Evolution 63:3258–3268
Rohlf FJ (1999) Shape statistics: Procrustes superimpositions and tangent spaces. J Classif 16:197–223
Rohlf FJ, Corti M (2000) Use of two-block partial least-squares to study covariation in shape. Syst Zool 49:740
Schiaffini MI, Segura V, Prevosti FJ (2019) Geographic variation in skull shape and size of the pampas fox Lycalopex gymnocercus (Carnivora: Canidae) in Argentina. Mammal Biol 97:50–58
Schlager S (2017) Morpho and Rvcg - shape analysis in R. In: Zheng G, Li S, Szekely G (eds) Statistical Shape and Deformation Analysis. Academic Press, London, pp 217–256
Schluter D (1996) Adaptive radiation along genetic lines of least resistance. Evolution 50:1766–1774
Segura V (2014) Ontogenia craneana postnatal en canidos y felidos neotropicales: funcionalidad y patrones evolutivos. Ph.D. Thesis, Universidad Nacional de La Plata, Argentina
Segura V, Cassini GH, Prevosti FJ (2017) Three-dimensional cranial ontogeny in pantherines (Panthera leo, P. onca, P. pardus, P. tigris; Carnivora: Felidae). Biol J Linnean Soc 120:210–227
Segura V, Prevosti FJ (2012) A quantitative approach to the cranial ontogeny of Lycalopex culpaeus (Carnivora: Canidae). Zoomorphology 131:79–92
Sidlauskas B (2008) Continuous and arrested morphological diversification in sister clades of characiform fishes: a phylomorphospace approach. Evolution 62:3135–3156
Silva FM, Prudente ALDC, Machado FA, Santos MM, Zaher H, Hingst-Zaher E (2017) Aquatic adaptations in a Neotropical coral snake: a study of morphological convergence. J Zool Syst Evol Res 4:393–313. https://doi.org/10.1111/jzs.12202
Slater GJ, Dumont ER, Van Valkenburgh B (2009) Implications of predatory specialization for cranial form and function in canids. J Zool 278:181–188. https://doi.org/10.1111/j.1469-7998.2009.00567.x
Slater GJ, Price SA, Santini F, Alfaro ME (2010) Diversity versus disparity and the radiation of modern cetaceans. Proc R Soc B-Biol Sci 277:3097–3104
Sydney NV, Machado FA, Hingst-Zaher E. (2012) Timing of ontogenetic changes of two cranial regions in Sotalia guianensis (Delphinidae). Mammal Biol 77:397–403
Therrien F (2005) Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators. J Zool 267:249–270
Tseng ZJ, Binder WJ (2009) Mandibular biomechanics of Crocuta crocuta, Canis lupus, and the late Miocene Dinocrocuta gigantea (Carnivora, Mammalia). Zool J Linnean Soc 158:683–696
Van Valkenburgh B (1991) Iterative evolution of hypercarnivory in canids (Mammalia: Carnivora): evolutionary interactions among sympatric predators. Paleobiology 17:340–362
Van Valkenburgh B, Wayne RK (1994) Shape divergence associated with size convergence in sympatric East African jackals. Ecology 75:1567–1581
Wainwright PC, Reilly SM (1994) Ecological Morphology. University of Chicago Press, Chicago
Wayne RK (1986) Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution 40 (2):243–261
Zrzavý J, Duda P, Robovský J, Okřinová I, Řičánková VP (2018) Phylogeny of the Caninae (Carnivora): combining morphology, behaviour, genes and fossils. Zool Scr 47:373–389
Zurano JP, Martinez PA, Canto-Hernandez J, Montoya-Burgos JI, Costa GC (2017) Morphological and ecological divergence in South American canids. J Biogeogr 44:821–833
Acknowledgments
We thank to N. Toledo and S. Vizcaíno for inviting us to participate in this tribute to L.B. Radinsky within the framework of the Symposium: El paradigma de correlación forma-función en mastozoología: un tributo a Leonard Radinsky (1937–1985), which took place during the XXXI Jornadas Argentinas de Mastozoología, in La Rioja, Argentina, 25 October, 2018. For permission to access material under their care and the attention in mammal collections we thank Robert Voss, Eileen Westwig (AMNH), Sergio Bogan, Yolanda Davies (CFA), Rubén Barquez, Mónica Díaz (CML), Bruce Patterson (FMNH), Mauro Lucherini, Estela Luengos (GECM), Mauro Schiaffini, Gabriel Martin (LIEB), Andrés Pautasso (MFAZV), Pablo Teta, David Flores, Sergio Lucero (MACN), Diego Verzi, Itatí Olivares (MLP), Mario de Vivo, Juliana Gualda (MZUSP), and Kristofer Helgen, Darrin Lunde (NMNH). This is a contribution to PICT 2014-1930, 2015-2389, 2015-0966, 2016-3151, 2016-3682, PUE 0125 and DEB 1350474 (NSF grant to Liam Revell).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Comment on Ethics
This article does not contain any studies with human participants or animals performed by any of the authors.
Appendix 1. List of specimens used in this study
Appendix 1. List of specimens used in this study
Atelocynus microtis (N = 23). AMNH: 76031; 76579; 95284; 95285; 98639; 100095. FMNH: 5249; 57836; 60674; 60675; 60676; 93955; 98080; 98081; 110949; 121286. MZUSP: 4320; 19750; 19751; 19752; 19753; 19754. NMNH: 361013.
Canis aureus (N = 1). MLP: 1035–1031.
Canis latrans (N = 1). MACN: 25.123.
Canis lupus (N = 5). AMNH: 18215. MACN 3.76; 23.15; 35.210. MLP: 1031.
Canis nehringi (N = 1). MACN-PV: 500.
Cerdocyon thous (N = 109). CFA: 3697; 3875; 4265; 4419; 4496; 4511; 4512; 4661; 4663; 4664; 4717; 5048; 5197; 5278; 5283; 5313; 5375; 6000; 6071; 6128; 6129. CML: 588; 3719; 3756; 3827; 4083; 4083; 4692; 5964; 5966; 5967; 6213; 6214; 6340. MACN: 4.213; 20.32; 24.85; 24.127; 25.119; 25.159; 29.839; 30.344; 30.345; 32.261; 32.262; 32.75; 33.6; 34.676; 36.191; 36.481; 39.460; 43.26; 44.11; 45.34; 45.40; 47.116; 47.189; 47.190; 47.191; 47.192; 47.193; 47.402; 48.3; 48.5; 48.6; 48.7; 48.10; 49.221; 49.367; 50.40; 50.43; 50.45; 50.57; 50.59; 50.60; 50.61; 50.62; 50.63; 50.64; 52.54; 52.63; 52.64; 13051; 14,322; 14681; 15741; 16189; 20316; 20454; 20456; 20815; 20816; 20817; 21228; 23180; 23669; 23670; 23726; 23727; 24045; 24046; 24207; 24208. MFA-ZV: 228; 1204. MLP: 20.IX.49.13; 16.X.01.7; 31.XII.02.77; 1322. MZUSP: 9687.
Chrysocyon brachyurus (N = 82). AMNH: 36962; 71179; 120999; 133940; 133941; 135274. CFA: 12826; 12827. CML: 1376; 6133; 6352. FMNH: 28311; 28312; 28313; 44534; 46003; 54406; 96003; 101848; 125401; 127434; 134483; 137425; 150739. MACN: 3.71; 3.73; 4.32; 4.303; 24.4; 30.29; 30.231; 53.49; 13466; 19146; 20646; 23456; 23984; 24043; 24201. MFA-ZV 517; 524; 581; 651; 652; 919; 1166. MLP: 2.IV.02.4; 5.X.99.1; 31.XII.02.88; 6; 92; 564; 695; 1684; 1686. MZUSP: 525; 3025; 3338; 9420; 19733; 19736; 29870; 31981; 32039; 32042; 32043; 32056; 32199; 32505; 32629. NMNH: 196975; 258614; 261022; 261023; 270371; 271567; 314863; 521007; 534807; 534970; 588,223; 588425.
Lycalopex culpaeus (N = 107). CFA: 2129; 6451. CML: 5067; 5068; 5069; 5070; 5071; 5970; 5974; 6343; 6344. LIEB: 791; 793. MACN: 3.68; 4.41; 7.42; 24.119; 25.128; 27.131; 30.69; 31.58; 31.59; 33.67; 33.68; 33.69; 38.39; 41.55; 15024; 15033; 15037; 15040; 15044; 15045; 15049; 15050; 15055; 15062; 15063; 15064; 15073; 15078; 15081; 15082; 15083; 15089; 15093; 15096; 15101; 15106; 15112; 15119; 15121; 15124; 15127; 15138; 15140; 15149; 15151; 15154; 15158; 15163; 15168; 15172; 15173; 15177; 15181; 15882; 15190; 15194; 15196; 15208; 15212; 15220; 15223; 15224; 15226; 15227; 15228; 15229; 15232; 15233; 15240; 15243; 15246; 15248; 19221; 19222; 20813; 21899; 23072; 23076; 23077; 23093; 23095; 23103; 23108; 23119; 23123; 23125; 23148; 23719; 23720; 23721; 23,915; 24210. MLP: 1264; 1266.
Lycalopex fulvipes (N = 2). FMNH: 23814; 23815.
Lycalopex griseus (N = 127). AMNH: 17440a; 17440b; 17441a. CFA: 2175; 4197; 5291; 5649; 5650; 5777; 5782; 10243. CML: 837; 838; 1177; 1178; 1427; 1489; 3714; 4967; 6189; 6190; 6192. FMNH: 154639; 154640. LIEB: 794; 809. MACN: 4.253; 23.20; 24.50; 24.52; 24.53; 24.54; 24.56; 24.57; 24.59; 24.62; 24.63; 24.64; 24.66; 24.68; 24.69; 24.71; 24.74; 24.75; 24.76; 24.79; 24.80; 24.81; 223; 225; 226; 13781; 14540; 14902; 15020; 15185; 15186; 15187; 15189; 15262; 15263; 15264; 15265; 15269; 16321; 16322; 16325; 20205; 20206; 20207; 20208; 20276; 20277; 20278; 20814; 20829; 23150; 23468; 23662; 23,663; 2664; 23668; 23718; 23728; 23729; 23730; 23910; 24206; 29.895; 50.419; 50.420; 50.432; 50.490; 51.170. MLP: 5.III.36.12; 5.III.36.27; 2.IV.60.1; 4.VIII.98.4; 240; 441; 559; 696; 701; 712. NMNH: 92139; 92140; 92141; 92142; 92143; 92144; 92145; 92146; 92147; 92149; 92150; 92151; 92152; 92169; 92173; 92174; 92175; 92176; 92177; 92178; 92179; 482163; 482164.
Lycalopex gymnocercus (N = 355). AMNH: 41502; 41503; 41504; 41505; 41506; 41507; 41508; 41509; 41510. CFA: 3255; 3698; 3962; 4256; 4406; 4416; 4417; 4659; 8312; 8313; 8588; 8589; 8590; 8591; 10887; 11062; 11063. CML: 192; 495; 545; 645; 834; 836; 895; 908; 909; 959; 1179; 1526; 1526; 3072; 4081; 4082; 5143; 5473; 5474; 5479; 5480; 5772; 6342. GECM: 24; 34; 40; 51; 57; 65; 67; 75; 76; 85; 100; 108; 112; 119; 121; 129; 139; 149; 152; 153; 179; 217Bis; 220Bis; 227Bis. MACN: 4.271; 20.33; 26.28; 20.35; 23.33; 23.34; 23.36; 23.37; 23.38; 24.48; 24.49; 24.133; 24.134; 24.140; 24.141; 24.142; 24.143; 24.144; 24.145; 24.146; 24.147; 24.148; 24.149; 24.151; 24.152; 24.154; 24.156; 24.162; 24.169; 24.170; 26.129; 26.162; 26.163; 27.53; 28.182; 29.35; 30.150; 30.210; 30.211; 30.212; 32.252; 32.263; 33.177; 33.266; 33.268; 34.317; 35.241; 36.178; 36.479; 36.480; 37.82; 38.243; 39.191; 39.194; 41.220; 41.221; 44.17; 48.266; 49.134; 49.139; 49.148; 49.149; 49.159; 49.160; 49.167; 50.56; 50.443; 50.491; 50.492; 50.494; 50.495; 50.497; 50.498; 50.500; 50.501; 50.502; 50.503; 50.504; 50.505; 51.81; 53.2; 54.133; 246; 285; 293; 13299; 13313; 13327; 13331; 13337; 14319; 14323; 14386; 14409; 15363; 15364; 15387; 15388; 15389; 15390; 15601; 15692; 15742; 15748; 15749; 15750; 15751; 15752; 15754; 15757; 15758; 15760; 15761; 15762; 15764; 15765; 1766; 157769; 15771; 15783; 15784; 15785; 15787; 15788; 15791; 15792; 15794; 15795; 15796; 15797; 15800; 15818; 15820; 15831; 15833; 15834; 15838; 15854; 15859; 15862; 15863; 15864; 15865; 15866; 15867; 15868; 15869; 15870; 15871; 15873; 15875; 15879; 15882; 15888; 15892; 15894; 15895; 15896; 15898; 15901; 15902; 15906; 15908; 15909; 15917; 15932; 15933; 15934; 15938; 15941; 15958; 15963; 15964; 15966; 15970; 15973; 15979; 15981; 15982; 15986; 15987; 15992; 15998; 15999; 16000; 16001; 16006; 16009; 16010; 16013; 16014; 16015; 16024; 16025; 16026; 16027; 16030; 16031; 16032; 16035; 16036; 16037; 16038; 16039; 16040; 16041; 16046; 16047; 16048; 16049; 16050; 16055; 16059; 16062; 16063; 16066; 16068; 16074; 16077; 16079; 16080; 16083; 16085; 16088; 16094; 16096; 16097; 16099; 16100; 16101; 16102; 16103; 16104; 16105; 16106; 16107; 16108; 16110; 16111; 16115; 16117; 16118; 16120; 16122; 16123; 16130; 16131; 16139; 16143; 16145; 16149; 16151; 22936; 23153; 23154; 23155; 23156; 23157; 23158; 23290; 23920; 24203; 24204; 24205; 24208; 24209; 24259; 24265; 24282. MLP: 16.III.99.16; 13.IV.99.3; 13.IV.99.13; 13.IV.99.14; 13.IV.99.36; 26.V.95.9; 4.VIII.98.9; 30.XII.02.65; 710. NMNH: 172789; 172790; 236366; 331065.
Lycalopex sechurae (N = 35). AMNH: 100091; 100100; 133926; 133927; 133928; 133929; 133937; 2091; 349; 36457; 391; 46525; 46526; 46527; 46528; 46529; 46530; 46531; 46532; 46533; 63709; 70091. FMNH: 19971; 19972; 20747; 53911; 80953; 80954; 80955; 80956; 80957; 80958; 80959; 80960; 80961; 80962; 80963; 80964; 80965; 80966; 80967; 80968; 80969.
Lycalopex vetulus (N = 31). MLP: 1258. MZUSP: 1011; 1012; 1014; 1015; 1016; 1018; 1075; 1076; 1084; 12040; 13611; 3046; 3047; 3047; 3048; 3049; 3050; 825. NMNH 121171; 121172; 181150; 545109.
Speothos venaticus (N = 34). AMNH: 136285; 167846; 175306; 184688; 37472; 76035; 76805; 76806; 98558; 98559; 98560; 98640. FMNH: 121544; 125402; 60290; 87861. MACN: 50.67; 16510. MZUSP:19743; 19744. NMNH: 253504; 270165; 270171; 270368; 270369; 270370; 307650; 314048; 395841; 398030; 521045; 538307; 544414; 582465.
Urocyon cinereoargenteus (N = 37). AMNH: 255645; 255648; 254470; 8197; 243449; 100301; 243095; 120989; 184105; 184122; 184012; 183939; 184002; 184065; 183979; 184098; 184094; 184077; 184091; 184126; 184083; 184121; 184014; 184087; 184013; 184009; 183991; 183956; 183942; 183943; 183960; 183953; 183995; 183954; 185512; 184064; 184007.
Vulpes lagopus (N = 1). MACN: 4.1.
Vulpes vulpes (N = 54). FMNH: 106726; 107271; 140172; 140176; 74472; 74987; 74988; 74989; 75644; 75645; 75646; 77130; 77136; 78650; 78651; 80827; 80829; 80836; 80837; 80839; 80840; 84697; 84698; 85216; 85217; 85218; 86820; 89369; 89370; 89371; 89372; 89587; 89710; 89712; 89963; 90361; 90473; 90474; 91605; 91725; 91726; 91731; 91741; 92727; 95863; 95865; 95867; 95870; 95872; 95873; 98733; 98734; 98735; 98736.
Vulpes zerda (N = 2). CML: 3731. MACN: 3.14.
Theriodictis platensis (N = 1). MLP-PV: 96-IX-1-1.
Otocyon megalotis (N = 1). MACN: 26.115.
Lycaon pictus (N = 1). MACN: 38.249.
Rights and permissions
About this article
Cite this article
Segura, V., Cassini, G.H., Prevosti, F.J. et al. Integration or Modularity in the Mandible of Canids (Carnivora: Canidae): a Geometric Morphometric Approach. J Mammal Evol 28, 145–157 (2021). https://doi.org/10.1007/s10914-020-09502-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-020-09502-z