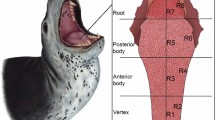
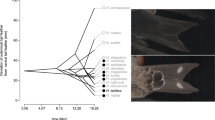
Abstract
Studies of functional morphology focus on species showing evident specializations, or at least some degree of divergence from the so-called “generalized phenotype.” This may have led to the proposal of the form-function correlation paradigm, which assumes that there is a close relationship between anatomical traits and their biological use. A question that arises in relation to digging is what minimum structural and functional requirements would be needed to carry it out properly regarding a particular biological role? Here, we review the main approaches and results achieved in the study of morphological traits related to the highly mechanically demanding function of digging, especially in the South American subterranean rodents Ctenomys and related caviomorph rodents within the mammalian context. It is seen that whatever the biological role that soil digging will play for a particular group of organisms, they must have the ability to disaggregate a relatively resistant material and, therefore, the magnitude of the produced force is a critical point. Muscles of diggers should not only be able to produce large forces but also to sustain them for long periods of time, for which the muscle architecture is another important issue. Digging tools (e.g., limb bones, claws, incisors, skull) must have the capacity to withstand the concomitant reaction forces without structural failure. In this regard cranial sutures, jaw and skull geometry could play an important role in force dissipation. Examples of species that do not diverge from the generalized surface-dweller phenotype, but are nonetheless capable of constructing complex burrows, are examined regarding some evolutionary scenarios where fulfilling minimum functional requirements would have allowed organisms to adequately perform new biological roles.







Similar content being viewed by others
References
Ade M, Ziekur I (1999) The forepaws of the rodents Cryptomys hottentotus (Bathyergidae) and Nannospalax ehrenbergi (Muridae, Spalacinae): phylogenetic and functional aspects. Mitt Mus Naturkd Berl in Zool Reihe 75:11–17
Álvarez GI, Díaz AO, Vassallo AI, Longo MV, Becerra, F (2012) Histochemical and morphometric analysis of the musculature of the forelimb of the subterranean rodent Anat Histol Embryol 41:317–325
Álvarez A, Vieytes EC, Becerra F, Olivares AI, Echeverría AI, Verzi DH, Vassallo AI (2015) Diversity of craniomandibular morphology in caviomorph rodents: an overview of macroevolutionary and functional patterns. In: Vassallo AI, Antenuchi CD (eds) Biology of Caviomorph Rodents: Diversity and Evolution. SAREM Sociedad Argentina para el Estudio de los Mamíferos, Buenos Aires, pp 199–228
Becerra F (2011) Aparato masticatorio en roedores caviomorfos (Rodentia, Hystricognathi): análisis morfo-funcional con énfasis en el género Ctenomys (Ctenomyidae). PhD dissertation, University of Mar del Plata
Becerra F, Casinos A, Vassallo AI (2013) Biting performance and skull biomechanics of a chisel tooth digging rodent (Ctenomys tuconax; Caviomorpha; Octodontoidea). J Exp Zool 319A:74–85
Becerra F, Echeverría AI, Casinos A, Vassallo AI (2014) Another one bites the dust: bite force and ecology in three caviomorph rodents (Rodentia, Hystricognathi). J Exp Zool 321A:220–232
Becerra F, Echeverría AI, Vassallo AI, Casinos A (2011) Bite force and jaw biomechanics in the subterranean rodent Talas tuco-tuco (Ctenomys talarum) (Caviomorpha: Octodontoidea). Can J Zool 89:334–342
Biknevicius A (1993) Biomechanical scaling of limb bones and differential limb use in caviomorph rodents. J Mammal 74:95–10
Blits KC (1999) Aristotle: form, function, and comparative anatomy. Anat Rec 257:58–63
Borges LR, Maestri R, Kubiak BB, Galiano D, Fornel R, Freitas TRO (2016) The role of soil features in shaping the bite force and related skull and mandible morphology in the subterranean rodents of genus Ctenomys (Hystricognathi: Ctenomyidae). J Zool 117:447–462
Buezas GN, Becerra F, Echeverría AI, Cisilino A, Vassallo AI (2019) Mandible strength and geometry in relation to bite force: a study in three caviomorph rodents. J Anat 234:564–575
Buezas GN, Becerra F, Vassallo AI (2017) Cranial sutural morphology in caviomorph rodents (Rodentia; Ctenohystrica). J Morphol 278:1125–1136
Campos CM, Tognelli MF, Ojeda RA (2001) Dolichotis patagonum. Mammal Species 625:1–5
Carrizo LV, Tulli MJ, Abdala V (2014) An ecomorphological analysis of forelimb musculotendinous system in sigmodontine rodents (Rodentia, Cricetidae, Sigmodontinae). J Mammal 95:843–854
Cohen M, Longo MV, Vassallo AI, Díaz AO (2017) Estudio histoquímico preliminar de los músculos tríceps longus y bíceps brachii del roedor epígeo Cavia aperea. XXX Jornadas Argentinas de Mastozoología. Bahía Blanca, 14-17 de Noviembre
Costa FR, Clerici GP, Rosa PS, Ribeiro LL, Rocha-Barbosa O (2017) Kinematic description of the vertical climbing of Dasypus novemcinctus (Xenarthra,Dasypodidae): the first report of this ability in armadillos. Mastozool Neotrop 24:451–456
Cox PG, Faulkes CG (2014) Digital dissection of the masticatory muscles of the naked mole-rat, Heterocephalus glaber (Mammalia, Rodentia). PeerJ 2:e448
Cox PG, Hautier L (eds) (2015) Evolution of the Rodents: Advances in Phylogeny, Functional Morphology and Development. Cambridge University Press, Cambridge
Cubo J, Ventura J, Casinos A (2006) A heterochronic interpretation of the origin of digging adaptations in the northern water vole, Arvicola terrestris (Rodentia: Arvicolidae). Biol J Linn Soc 87:381–391
Currey JD (2002) Bone: Structure and Mechanics. Princeton University Press, Princeton
Echeverría AI (2011) Ontogenia del comportamiento en el roedor subterráneo Ctenomys talarum (Rodentia: Ctenomyidae). PhD dissertation, University of Mar del Plata
Echeverría AI, Abdala V, Longo MV, Vassallo AI (2019) Functional morphology and identity of the thenar pad in the subterranean genus Ctenomys (Rodentia, Caviomorpha). J Anat 235:940–952
Echeverría AI, Becerra F, Vassallo AI (2014) Postnatal ontogeny of limb proportions and functional indices in the subterranean rodent Ctenomys talarum (Rodentia: Ctenomyidae). J Morphol 275:902–913
Echeverría AI, Biondi LM, Becerra F, Vassallo AI (2016) Postnatal development of subterranean habits in tuco-tucos Ctenomys talarum (Rodentia, Caviomorpha, Ctenomyidae) J Ethol 34:107-118
Elissamburu A (2004) Análisis morfométrico y morfofuncional del esqueleto apendicular de Paedotherium (Mammalia, Notoungulata). Ameghiniana 41:363–380
Elissamburu A, Vizcaíno SF (2004) Limb proportions and adaptations in caviomorph rodents (Rodentia: Caviomorpha). J Zool 262:145–159
Ercoli MD, Álvarez A, Stefanini MI, Busker F, Morales MM (2015) Muscular anatomy of the forelimbs of the lesser grison (Galictis cuja), and a functional and phylogenetic overview of Mustelidae and other Caniformia. J Mammal Evol 22:57–91
Eyal S, Rubin S, Krief S, Levin L, Zelzer E (2019) Common cellular origin and diverging developmental programs for different sesamoid bones. Development 146. doi:https://doi.org/10.1242/dev.167452
Fernández ME, Vassallo AI, Zárate M (2000) Functional morphology and paleobiology of the Pliocene rodent Actenomys (Caviomorpha: Octodontidae): the evolution to a subterranean mode of life. Biol J Linn Soc 71:71–90
Gambaryan PP, Gasc JP, Renous S (2002) Cinefluorographical study of the burrowing movements in the common mole, Talpa europaea (Lipotyphla, Talpidae). Russ J Theriol 1:91–109
Gomes Rodrigues H, Sumbera R, Hautier L (2016) Life in burrows channelled the morphological evolution of the skull in rodents: the case of African mole-rats (Bathyergidae, Rodentia). J Mammal Evol 23:175–189
Haffner M (1998) A comparison of the gross morphology and micro-anatomy of the foot pads in two fossorial and two climbing rodents (Mammalia). J Zool 244:287–294
Hautier L, Lebrun R, Saksiri S, Michaux J, Vianey-Liaud M, Marivaux L (2011) Hystricognathy vs sciurognathy in the rodent jaw: a new morphometric assessment of hystricognathy applied to the living fossil Laonastes (Diatomyidae). PLoS One 6:e18698.
Herring SW (2010) Muscle-bone interactions and the development of skeletal phenotype. In: Hallgrimsson B, Hall BK (eds) Epigenetics. University of California Press, Berkeley, pp 221–237
Herring SW, Teng S (2000) Strain in the braincase and its sutures during function. Am J Phys Antropol 112:575–593
Hickman GC (1985) Surface-mound formation by the tuco-tuco, Ctenomys fulvus (Rodentia: Ctenomyidae), with comments on earth-pushing in other fossorial mammals. J Zool 205:385–390
Hildebrand M (1985) Digging in quadrupeds. In: Hildebrand M, Bramble DM, Liem KF, Wake DB (eds) Functional Vertebrate Morphology. Belknap Press, Cambridge, pp 89–109
Hildebrand M, Bramble DM, Liem KF, Wake DB (eds) (1985) Functional Vertebrate Morphology. Belknap Press, Cambridge
Jaslow CR (1990) Mechanical properties of cranial sutures. J Biomech 23:313–321
Kley NJ, Kearney M (2007) Adaptations for digging and burrowing. In: Hall BK (ed) Fins into Limbs: Evolution, Development, and Transformation. University of Chicago Press, Chicago, pp 284–309
Laland KN, Odling-Smee J, Gilbert SF (2008) EvoDevo and niche construction: building bridges. J Exp Zool B Mol Dev Evol 310:549–566
Lehman WH (1963) The forelimb architecture of some fossorial rodents. J Morphol 113:59–75
Lessa EP, Stein BR (1992) Morphological constraints in the digging apparatus of pocket gophers (Mammalia, Geomyidae). Biol J Linn Soc 47:439–453
Lessa EP, Vassallo AI, Verzi DH, Mora MS (2008) Evolution of morphological adaptations for digging in living and extinct ctenomyid and octodontid rodents. Biol J Linn Soc 95:267–283
Longo MV, Díaz AO (2013) The claw closer muscle of two estuarine crab species, Cyrtograpsus angulatus and Neohelice granulata (Grapsoidea, Varunidae): histochemical fibre type composition. Acta Zool 94:233–239
Longo MV, Díaz AO, Goldemberg AL (2011) The claw closer muscle of Neohelice granulata (Grapsoidea, Varunidae): a morphological and histochemical study. Acta Zool 92:126–133
Longo MV, Díaz AO, Vassallo AI (2017) Morfología funcional de la musculatura masetérica del roedor subterráneo Ctenomys talarum: histoquímica de tipos de fibras. XXX Jornadas Argentinas de Mastozoología. Bahía Blanca, 14-17 de Noviembre
Marroig G, Cheverud JM (2001) A comparison of phenotypic variation and covariation patterns and the role of phylogeny, ecology, and ontogeny during cranial evolution of New World monkeys. Evolution 55:2576–2600
Mayr E (1963) Animal Species and Evolution. Harvard University Press, Cambridge
McIntosh AF, Cox PG (2016) The impact of gape on the performance of the skull in chisel-tooth digging and scratch digging mole-rats (Rodentia: Bathyergidae). R Soc Open Sci 3(10): 160568
Menegaz RA, Sublett SV, Figueroa SD, Hoffman TJ, Ravosa MJ, Aldridge K (2010) Evidence for the influence of diet on cranial form and robusticity. Anat Rec 293:630–641
Mora MS, Becerra F, Vassallo AI (2018) An allometric analysis of sexual dimorphism in Ctenomys australis: integrating classic morphometry and functional performance in vivo. Zoology 127:27–39
Morgan CC, Verzi DH (2006) Morphological diversity of the humerus of the South American subterranean rodent Ctenomys (Rodentia, Ctenomyidae). J Mammal 87:1252–1260
Napier JR (1980) Hands. Princeton University Press, Princeton
Ojeda AA, Tarquino-Carbonell A, Veléz LM, Ojeda RA (2018) Tympanoctomys: 75 años de historia. Estado actual del género y perspectivas futuras. Rev Mus Argentino Cienc Nat 20:109–122
Ozkaya N, Nordin M (1999) Fundamentals of Biomechanics. Springer Verlag, Berlin
Pardiñas UFJ, Galliari CA (2001) Reithrodon auritus. Mammal Species 664:1–8
Pedersen SC (2000) Skull growth and the acoustical axis of the head in bats. In: Adams RA, Pedersen SC (eds) Ontogeny, Functional Ecology, and Evolution of Bats. Cambridge University Press, Cambridge, pp 174–213
Pérez EM (1992) Agouti paca. Mammal Spec 404:1–7
Pérez MJ, Barquez RM, Díaz MM (2017) Morphology of the limbs in the semi-fossorial desert rodent species of Tympanoctomys (Octodontidae, Rodentia). Zoo Keys 710:77–96
Radinsky LB (1984) Ontogeny and phylogeny in horse skull evolution. Evolution 38:1–15
Radinsky LB (1985a) Approaches in evolutionary morphology: a search for patterns. Annu Rev Ecol Syst 16:1–14
Radinsky LB (1985b) Patterns in the evolution of unguate jaw shape. Am Zool 25:303–314
Rayfield E (2007) Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annu Rev Earth Planet Sci 35:541–576
Redford KH, Eisenberg JF (1992) Mammals of the Neotropics. Vol. 2. The South Cone: Chile, Argentina, Uruguay, Paraguay. University of Chicago Press, Chicago
Reichman OJ, Smith SC (1990) Burrows and burrowing behavior by mammals. In: Genoways HH (ed) Current Mammalogy. Plenum Press, New York, pp 197–244
Segura V (2015) A three-dimensional skull ontogeny in the bobcat (Lynx rufus) (Carnivora: Felidae): a comparison with other carnivorans. Can J Zool 93:225–237
Smith KK (1993) The form of the feeding apparatus in terrestrial vertebrates: Studies of adaptation and constraint. In: Hanken J, Hall BK (eds) The Skull - Functional and Evolutionary Mechanisms, Vol 3. University of Chicago Press, Chicago, pp 150–196
Soons J, Herrel A, Genbrugge A, Aerts P, Podos J, Adriaens D, de Witte Y, Jacobs P, Dirckx J (2010) Mechanical stress, fracture risk and beak evolution in Darwin’s ground finches (Geospiza). Philos Trans R Soc B 365:1093–1098
Stein BR (2000) Morphology of subterranean rodents. In: Lacey EA, Patton JL, Cameron GN (eds) Life Underground: The Biology of Subterranean Rodents. University of Chicago Press, Chicago, pp 19–61
Tavares WC, Vozniak JH, Pessôa LM (2019) Evolution of appendicular specializations for fossoriality in euryzygomatomyine spiny rats across different Brazilian biomes (Echimyidae, Hystricognathi, Rodentia). J Mammal Evol https://doi.org/10.1007/s10914-019-09459-8
Tognelli M, Campos C, Ojeda RA (2001) Microcavia australis. Mammal Species 648:1–4
Ubilla M (2008) Postcranial morphology of the extinct caviine rodent Microcavia criolloensis (late Pleistocene, South America). Zool J Linn Soc 154:795–806
Ubilla M, Altuna C (1990) Analyse de la morphologie de la main chez des espèces de Ctenomys de l’Uruguay (Rodentia, Octodontidae): adaptations au fouissage et implications évolutives. Mammalia 54:108–117
Van Daele PA, Herrel A, Adriaens D (2009) Biting performance in teeth-digging African mole-rats (Fukomys, Bathyergidae, Rodentia). Physiol Biochem Zool 82:40–50
Van Wassenbergh S, Heindryckx S, Adriaens D (2017) Kinematics of chisel-tooth digging by African mole-rats. J Exp Biol 220:4479–4485
Vassallo AI (1998) Functional morphology, comparative behavior, and adaptation in two sympatric subterranean rodent genus Ctenomys (Rodentia: Octodontidae). J Zool 244:415–427
Vassallo AI, Becerra F, Echeverría AI, Casinos A (2015) Ontogenetic integration between force production and force reception: a case study in Ctenomys (Rodentia: Caviomorpha). Acta Zool 97:232–240
Vassallo AI, Mora MS (2007) Interspecific scaling and ontogenetic growth patterns of the skull in living and fossil ctenomyid and octodontid rodents (Caviomorpha: Octodntoidea). In: Kelt DA, Lessa EP, Salazar-bravo J, Patton JL (eds) The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson. Univ Calif Publ Zool 134:945–968
Verzi DH, Álvarez A, Olivares AI, Morgan CC, Vassallo AI (2010) Ontogenetic trajectories of key morphofunctional cranial traits in South American subterranean ctenomyid rodents. J Mammal 91:1508–1516
Vieytes EC, Morgan CC, Verzi DH (2007) Adaptive diversity of incisor enamel microstructure in South American burrowing rodents (family Ctenomyidae, Caviomorpha). J Anat 211:296–302
Vizcaíno SF, Fariña RA, Mazzetta GV (1999) Ulnar dimensions and fossoriality in armadillos. Acta Theriol 44:309–320
Wake MH (1993) The skull as a locomotor organ. In: Hanken J, Hall BK (eds) The Skull: Functional and Evolutionary Mechanisms. University of Chicago Press, Chicago, pp 197–240
Weber JN, Peterson BK, Hoekstra HE (2013) Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature 493:402–406
Weijs WA, Brugman P, Klok EM (1987) The growth of the skull and jaw muscles and its functional consequences in the New Zealand rabbit (Oryctolagus cuniculus). J Morphol 194:143–161
Woods CA (1972) Comparative myology of jaw, hyoid and pectoral appendicular regions of new and Old World hystricomorph rodents. Bull Am Mus Nat Hist 147:119–198
Acknowledgements
We thank Drs. S. Vizcaíno, G. Cassini, and N. Toledo for the invitation to participate in the symposium “The paradigm of shape-function correlation in mastozoology: a tribute to Leonard Radinsky,” which was held in La Rioja during the XXXI Jornadas Argentinas de Mastozoología. We thank Joshua Asel who kindly provided the photo of the rata vizcacha colorada (Tympanoctomys barrerae). Financial support: CONICET PIP 2014-2016 N ° 11220130100375 and Grant EXA918 / 18 from University of Mar del Plata.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vassallo, A.I., Becerra, F., Echeverría, A.I. et al. Analysis of the Form-Function Relationship: Digging Behavior as a Case Study. J Mammal Evol 28, 59–74 (2021). https://doi.org/10.1007/s10914-019-09492-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-019-09492-7