Abstract
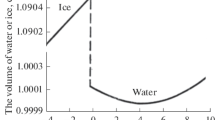
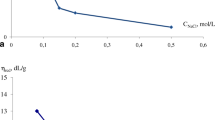
The water-structural aspects of evolution of interphase nanoformations are considered. Monitoring the state of nanoformations provides the control of hydrophobic interaction and flotation macroprocesses.
Similar content being viewed by others
References
Yu. I. Petrov, Clusters and Small Particles [in Russian], Nauka, Moscow (1986).
G. S. Hodakov, Physics of Grinding [in Russian], Nauka, Moscow (1972).
V. I. Klassen, Problems of Aeration and Flotation Theory [in Russian], Goskhimizdat, Moscow (1949).
I. N. Plaksin, R. Sh. Shafeev, and V. A. Chanturia, Effect of Mineral Surface Heterogeneity on Interaction with Flotation Reagents [in Russian], Nauka, Moscow (1965).
D. N. Abishev and Yu. P. Eremin, “Finely-disseminated ore processing is a priority direction of mining and smelting complex,” Promeshlenost Kazakhstana, No. 2 (2000).
V. E. Vigdergauz, “Kinetics of bubble — particle interaction, surface hydrophobicity and interfacial phenomena in sulphide mineral flotation,” in: Centenary of Flotation Proceedings, AIMM, Brisbane (2005).
N. S. Bekturganov, Yu. P. Eremin, A. A. Zharmenov, and V. G. Zagainov, “Nanotechnological aspects of flotation process,” Promyshlennost Kazakhstana, No. 1 (2006).
A. F. Taggart, Handbook of Ore Dressing, John Wiley and Sons, New-York (1927).
A. M. Gaudin, Flotation, Mc.Graw-Hill Book Company, New-York (1932).
K. L. Satherland and I. W. Work, Principles of Flotation, AIMM, Mellown (1955).
I. Langmuir, “The structure of molecular groups formed on surfaces of liquids,” Proc. Nat. Acad. Sci., 3 (1917).
S. I. Mitrophanov, Selective Flotation [in Russian], Moscow (1958).
G. Nemethy, and H. Scheraga, G. Chem. Phisica, 36, No. 12 (1962).
V. I. Revnivtsev, Yu. P. Eremin, V. A. Glembotskii, G. A. Denisov, and N. N. Havskii, “The USSR Scientific Discovery No. 370. Phenomenon of pre-hypersonic activation of interdisperse interactions in aqueous systems,” in: USSR State Register of Discoveries [in Russian], September 14, 1989 with the priority from January 20, 1972.
G. S. Berger, N. K. Omarova, M. T. Baimakhanov, and E. G. Pershikova, “On role of hydrophobic interactions for the control of mineral floatability under pulp heating,” Izv. Vuzov, Tsvet. Met., No.5 (1978).
V. A. Chanturia and R. Sh. Shafeev, Chemistry of Surface Phenomena under Flotation [in Russian], Nedra, Moscow (1977).
V. A. Chanturia and V. E. Vigdergauz, Electrochemistry of Sulphides/Theory and Practice of Flotation [in Russian], Nauka, Moscow (1993).
V. A. Chanturia, V. E. Vigdergauz, T. V. Nedosekina, M. V. Panova, and N. K. Gromova, “ Electrochemical study of wettability of sulphide minerals under flotation conditions: pyrite, pyrrhotite, and arsenopyrite,” Fiz.-Tekh. Probl. Razrab. Polezn. Iskop., No. 3 (1997).
V. A. Glembotskii and Yu. P. Eremin, “On effect of ultrasonic treatment on the modification of bubble surface properties in elementary flotation,” Dokl Akad Nauk SSSR, 183, No. 4 (1968).
L. Poling, General Chemistry [Russian translation], Mir, Moscow (1974).
Yu. P. Eremin, A. A. Zharmenov, V. A. Chanturia, V. E. Vigdergauz, N. S. Bektuganov, et al., “ Theory and technology of processing of natural and technogenic mineral materials,” in: Complex Processing of Mineral Raw Materials in Kazakhstan (State, Problems, and Solutions) [in Russian], 2, Foliant, Astana (2003).
G. Nemethy, “Hydrophobe Wechselwirkungen,” Angew. Chem., 79 (1967).
H. A. Scheraga, Protein Structure. Acad. Press, New-York, London (1961).
G. Shuster, Determinate Chaos [Russian translation], Mir, Moscow (1989).
Yu. L. Klimantovich, Statistical Theory of Open Systems [in Russian], 1, Yanus, Moscow (1995), 2, Yanus, Moscow (1999).
Yu. A. Dyadin and K. A. Udachin, “Clatratic polyhydrates of peralkylated salts and their analogues,” Zh. Strukt. Khim., 28, No. 3 (1987).
Yu. P. Eremin, Inclusion Phenomena and Hydrophobic Interactions./ Inclusion Compounds [in Russian], Izd. SB RAS, Novosibirsk (1989).
S. P. Gauda, Bound Water. Facts and Hypotheses [in Russian], Nauka, Novosibirsk (1982).
A. A. Abramov, “Theoretical analysis of formation of sorption layer of anion collector and hydrophobization of mineral surface,” Tsvet. Met., No. 11 (2005).
R. Horn, Marine Chemistry / Water Structure and Hydrosphere Chemistry [Russian translation], Mir, Moscow (1972).
Author information
Authors and Affiliations
Additional information
The pubication is a pilot scheme.
__________
Translated from Fiziko-Tekhnicheskie Problemy Razrabotki Poleznykh Iskopaemykh, No. 2, pp. 99–113, March–April, 2007.
Rights and permissions
About this article
Cite this article
Vigdergauz, V.E., Eremin, Y.P., Zharmenov, A.A. et al. Interphase layers of water-heterogeneous flotation systems as hydrated nanoassociates. J Min Sci 43, 198–211 (2007). https://doi.org/10.1007/s10913-007-0022-6
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10913-007-0022-6