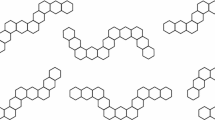
The total energy of all π-electrons in a conjugated hydrocarbon (within the framework of HMO approximation) is the sum of the absolute value of all the eigenvalues of its corresponding graph. In this paper, we consider “double hexagonal chains” as benzenoids constructed by successive fusions of successive naphthalenes along a zig–zag sequence of triples of edges as appear on opposite sides of each naphthalene unit. It is shown that if the fusions are such as to give a polyaceacene then the total π-electron energy is the minimum from among all the double hexagonal chains with the same number of naphthalene units.
Similar content being viewed by others
References
Gutman I., Polansky O.E. (1986). Mathematical concepts in organic chemistry. Springer, Berlin
Gutman I., Cyvin S.J. (eds) (1990). Advances in the Theory of benzenoid hydrocarbons. Topi. Curr. Chem., Vol. 153. Springer, Berlin
Gutman I. (eds) (1992). Advances in the Theory of benzenoid hydrocarbons I. Top. Curr. Chem., Vol. 162. Springer, Berlin
Gutman I. (1977). Theoret. Chim. Acta (Berlin) 45: 79
Gutman I., Hou Y. (2001). MATCH – Commun. Math. Comput. Chem. 43: 17
Hou Y. (2001). J. Math. Chem. 29: 163
Hou Y. (2002). Lin. Multilin. Algebra 49: 347
Li H. (1999). J. Math. Chem. 25: 145
Zhang F., Li H. (1999). Discrete Appl. Math. 92: 71
Zhang F., Li Z., Wang L. (2001). Chem. Phys. Lett. 337: 125
Zhang F., Li Z., Wang L. (2001). Chem. Phys. Lett. 337: 131
Seitz W.A., Klein D.J. (eds) (1985). Chem. Phys. Lett. 115: 139.
Klein D.J., Schmalz T.G. (eds) (1985). Chem. Phys. Lett. 120: 367.
Cvetkovic D.M., Doob M., Gutman et al. I. (1988). Ann. Discrete Math. 36: 50
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, H., Zhang, F. Double hexagonal chains with minimal total π-electron energy. J Math Chem 42, 1041–1056 (2007). https://doi.org/10.1007/s10910-006-9159-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-006-9159-9