Abstract
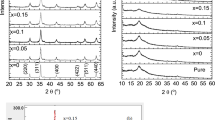
Ferrogels based on polyvinylpyrrolidone/polyvinyl alcohol (PVP/PVA) matrices with Mn0.6Fe2.4O4–polyethylene glycol (Mn0.6Fe2.4O4–PEG) were synthesized using the freezing–thawing method. The phase structure and morphology of ferrogels with Mn0.6Fe2.4O4–PEG filler were characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM), respectively. The Mn0.6Fe2.4O4–PEG/PVP/PVA ferrogel was characterized using small-angle X-ray scattering to determine the distribution of Mn0.6Fe2.4O4–PEG nanoparticles through two-lognormal data analysis; the semicrystallite distribution of the PVP/PVA was investigated using the Beaucage and Teubner–Strey models. The optical and electrical properties of the Mn0.6Fe2.4O4–PEG nanoparticles were characterized using a UV–Vis spectrophotometer. The XRD analysis showed that the Mn0.6Fe2.4O4–PEG exhibits an inverse-spinel cubic structure with an average particle size of 10.2 nm. This result was corroborated by TEM analysis, which revealed an average size of 10.9 nm through the Image-J software analysis. The two-lognormal method was used to analyze the distribution of Mn0.6Fe2.4O4–PEG nanoparticles in the ferrogel, revealing a secondary particle size of approximately 9.8 nm. These secondary particles are, on average, evenly arranged with respect to the primary particles with a diameter of 3.3 nm. UV–Vis data analysis showed that the refractive index and energy gap of the Mn0.6Fe2.4O4–PEG nanoparticles were approximately 2.79 and 2.24 eV, respectively. The optical conductivity and electrical conductivity calculated from the refractive-index and energy-gap data were 1.27 × 108 and 70 Ω−1 cm−1, respectively. These results indicate that the Mn0.6Fe2.4O4–PEG nanoparticles exhibit strong potential for use as a base material in optoelectronics applications.









Similar content being viewed by others
References
H. Oliveira, E. Pérez-Andrés, J. Thevenot, O. Sandre, E. Berra, S. Lecommandoux, Magnetic field triggered drug release from polymersomes for cancer therapeutics. J. Control. Release 169(3), 165–170 (2013)
L. Polo-corrales, J.E. Ramirez-vick, E.J. Hernandez-ramos, Thermosensitive hydrogels with nanofillers incorporated to use in food packaging applications. Int. J. Appl. Eng. Res. 13(10), 7305–7308 (2018)
F.A. Blyakhman et al., Polyacrylamide ferrogels with embedded maghemite nanoparticles for biomedical engineering. Results Phys. 7, 3624–3633 (2017)
S. Klein et al., Enhanced in vitro biocompatibility and water dispersibility of magnetite and cobalt ferrite nanoparticles employed as ros formation enhancer in radiation cancer therapy. Small 14(21), 1–10 (2018). https://doi.org/10.1002/smll.201704111
S.M. Taimoory et al., The synthesis and characterization of a magnetite nanoparticle with potent antibacterial activity and low mammalian toxicity. J. Mol. Liq. 265, 96–104 (2018). https://doi.org/10.1016/J.MOLLIQ.2018.05.105
Y. Fei et al., Aqueous superparamagnetic magnetite dispersions with ultra-high initial magnetic susceptibilities. Langmuir (2017). https://doi.org/10.1021/acs.langmuir.7b03702
Y. Du, J. Mao, Effect of magnetite nanoparticles on nuclear magnetic resonance imaging. J. Nanoelectron. Optoelectron. 12(9), 961–965 (2017). https://doi.org/10.1166/jno.2017.2224
M. Abdellahi, E. Karamian, A. Najafinezhad, F. Ranjabar, A. Chami, A. Khandan, Diopside-magnetite; a novel nanocomposite for hyperthermia applications. J. Mech. Behav. Biomed. Mater. 77, 534–538 (2018). https://doi.org/10.1016/j.jmbbm.2017.10.015
M. Gerosa, C.E. Bottani, C. Di Valentin, G. Onida, G. Pacchioni, Accuracy of dielectric-dependent hybrid functionals in the prediction of optoelectronic properties of metal oxide semiconductors: a comprehensive comparison with many-body GW and experiments. J. Phys. Condens. Matter. (2018). https://doi.org/10.1088/1361-648X/aa9725
S.P. Zustiak, J.B. Leach, Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromol 11(5), 1348–1357 (2010). https://doi.org/10.1021/bm100137q
Sunaryono, M.F. Hidayat, C. Insjaf, A. Taufiq, N. Mufti, Munasir, Investigation of magnetic properties and mechanical responses on hydrogel-TMAH-magnetite, IOP conference series: materials science and engineering, vol. 367 (IOP Publishing, Bristol, 2018). https://doi.org/10.1088/1757-899X/367/1/012025
L. Jiao, P. Qi, Y. Liu, B. Wang, L. Shan, Fe3O4 Nanoparticles embedded sodium alginate/PVP/calcium gel composite for removal of Cd2+. J. Nanomater. (2015). https://doi.org/10.1155/2015/940985
Y. Shi, D.S. Xiong, Y. Peng, N. Wang, Effects of polymerization degree on recovery behavior of PVA/PVP hydrogels as potential articular cartilage prosthesis after fatigue test. Express Polym. Lett. 10, 125–138 (2016)
G. Lu et al., Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 4(4), 310–316 (2012). https://doi.org/10.1038/nchem.1272
J.S. Gonzalez, P. Nicolás, M.L. Ferreira, M. Avena, V.L. Lassalle, V.A. Alvarez, Fabrication of ferrogels using different magnetic nanoparticles and their performance on protein adsorption. Polym. Int. 63(2), 258–265 (2014). https://doi.org/10.1002/pi.4498
A. Radoń, A. Drygała, Ł. Hawełek, D. Łukowiec, Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 131, 148–156 (2017). https://doi.org/10.1016/j.matchar.2017.06.034
A. Bahadur et al., Eco-friendly synthesis of magnetite (Fe3O4) nanoparticles with tunable size: dielectric, magnetic, thermal and optical studies. Mater. Chem. Phys. 198, 229–235 (2017). https://doi.org/10.1016/j.matchemphys.2017.05.061
S. Güner, M. Amir, M. Geleri, M. Sertkol, A. Baykal, Magneto-optical properties of Mn3+ substituted Fe3O4 nanoparticles. Ceram. Int. 41, 1–8 (2015). https://doi.org/10.1016/j.ceramint.2015.05.034
M. Cabuk, B. Gündüz, Controlling the optical properties of polyaniline doped by boric acid particles by changing their doping agent and initiator concentration. Appl. Surf. Sci. 424, 345–351 (2017). https://doi.org/10.1016/j.apsusc.2017.03.010
P. Jayakrishnan, M.T. Ramesan, Synthesis, characterization, electrical conductivity and material properties of magnetite/polyindole/poly(vinyl alcohol) blend nanocomposites. J. Inorg. Organomet. Polym. Mater. 27(1), 323–333 (2017). https://doi.org/10.1007/s10904-016-0474-8
S. Bagheli, H.K. Fadafan, R.L. Orimi, M. Ghaemi, Synthesis and experimental investigation of the electrical conductivity of water based magnetite nanofluids. Powder Technol. 274, 426–430 (2015). https://doi.org/10.1016/j.powtec.2015.01.050
B.Y. Yu et al., Assembly of magnetite nanoparticles into spherical mesoporous aggregates with a 3-D Wormhole-Like porous structure. J. Mater. Chem. 38, 8320–8328 (2010)
Sunaryono, Contributions of TMAH surfactant on hierarchical structures of PVA/Fe3O4–TMAH Ferrogels by using SAXS instrument.J. Inorg. Organomet. Polym Mater. https://doi.org/10.1007/s10904-018-0939-z
P.T. Bui, J.-H. Song, Z.-Y. Li, M.S. Akhtar, O.-B. Yang, Low temperature solution processed Mn3O4 nanoparticles: Enhanced performance of electrochemical supercapacitors. J. Alloys Compd. 694, 560–567 (2017)
X. Yang, J. Kan, F. Zhang, M. Zhu, S. Li, Facile fabrication of Mn2+ doped magnetite microspheres as efficient electrode material for supercapacitors. J. Inorg. Organomet. Polym Mater. 27(2), 542–551 (2017). https://doi.org/10.1007/s10904-017-0496-x
R. Otero-Lorenzo, E. Fantechi, C. Sangregorio, V. Salgueiriño, Solvothermally driven mn doping and clustering of iron oxide nanoparticles for heat delivery applications. Chemistry A 22(19), 6666–6675 (2016)
L.B. de Mello, L.C. Varanda, F.A. Sigoli, I.O. Mazali, Co-precipitation synthesis of (Zn–Mn)-co-doped magnetite nanoparticles and their application in magnetic hyperthermia. J. Alloys Compd. (2019). https://doi.org/10.1016/j.jallcom.2018.11.280
A. Taufiq et al., Nanoscale clustering and magnetic properties of MnxFe3−xO4 particles prepared from natural magnetite. J. Supercond. Novel Magn. 28(9), 2855–2863 (2015). https://doi.org/10.1007/s10948-015-3111-9
J. Teixeira, Small-angle scattering by fractal systems. J. Appl. Crystallogr. 21(6), 781–785 (1988). https://doi.org/10.1107/S0021889888000263
S.M. Yusuf et al., Structural and magnetic properties of amorphous iron oxide. Phys. B 405(4), 1202–1206 (2010). https://doi.org/10.1016/j.physb.2009.11.040
P.J. Vikesland, R.L. Rebodos, J.Y. Bottero, J. Rose, A. Masion, Aggregation and sedimentation of magnetite nanoparticle clusters. Environ. Sci: Nano 3(3), 567–577 (2016). https://doi.org/10.1039/c5en00155b
X. Lasheras et al., Mn-Doping level dependence on the magnetic response of MnxFe3–xO4 ferrite nanoparticles. Dalton Trans. 48(30), 11480–11491 (2019)
F.L. Deepak et al., A systematic study of the structural and magnetic properties of Mn-, Co-, and Ni-doped colloidal magnetite nanoparticles. J. Phys. Chem. C 119(21), 11947–11957 (2015). https://doi.org/10.1021/acs.jpcc.5b01575
J.M. Orozco-Henao et al., Effects of nanostructure and dipolar interactions on magnetohyperthermia in iron oxide nanoparticles. J. Phys. Chem. C 120(23), 12796–12809 (2016). https://doi.org/10.1021/acs.jpcc.6b00900
W. Szczerba, R. Costo, S. Veintemillas-Verdaguer, M. DelPuerto Morales, A.F. Thünemann, SAXS analysis of single-and multi-core iron oxide magnetic nanoparticles. J. Appl. Crystallogr. 50, 481–488 (2017). https://doi.org/10.1107/S1600576717002370
Sunaryono et al., Small-angle X-ray scattering study on PVA/Fe3O4 magnetic hydrogels. NANO. 11, 1650027 (2016). https://doi.org/10.1142/S1793292016500272
T. Puspitasari, K.M.L. Raja, D.S. Pangerteni, A. Patriati, E.G.R. Putra, Structural organization of poly(vinyl alcohol) hydrogels obtained by freezing/thawing and γ-irradiation processes: a Small-Angle Neutron Scattering (SANS) Study. Proced. Chem. 4, 186–193 (2012). https://doi.org/10.1016/j.proche.2012.06.026
O. Owoseni et al., Microstructural characteristics of surfactant assembly into a gel-like mesophase for application as an oil spill dispersant. J. Colloid Interface Sci. 524, 279–288 (2018)
M.M.N. Ansari, S. Khan, Structural, electrical and optical properties of sol-gel synthesized cobalt substituted MnFe2O4 nanoparticles. Phys. B 520, 21–27 (2017). https://doi.org/10.1016/j.physb.2017.06.020
A.S. Ahmed, M.L. Singla, S. Tabassum, A.H. Naqvi, A. Azam, Band gap narrowing and fluorescence properties of nickel doped SnO2 nanoparticles. J. Lumin. 131(1), 1–6 (2011). https://doi.org/10.1016/J.JLUMIN.2010.07.017
M. Khairy, M.E. Gouda, Electrical and optical properties of nickel ferrite/polyaniline nanocomposite. J. Adv. Res. 6(4), 555–562 (2015). https://doi.org/10.1016/j.jare.2014.01.009
J. Tauc, R. Grigorovici, A. Vancu, Optical properties and electronic structure of amorphous germanium. Phys Status Solidi (b) 15(2), 627–637 (1966)
P. Iranmanesh, S. Saeednia, M. Mehran, S.R. Dafeh, Modified structural and magnetic properties of nanocrystalline MnFe2O4by pH in capping agent free co-precipitation method. J. Magn. Magn. Mater. 425, 31–36 (2017). https://doi.org/10.1016/j.jmmm.2016.10.105
Muzammil et al., Effect of template on structural and band gap behaviors of magnetite nanoparticles. J. Phys.: Conf. Ser. 1093, 012020 (2018). https://doi.org/10.1088/1742-6596/1093/1/012020
A. Mary Jacintha, A. Manikandan, K. Chinnaraj, S. Arul Antony, P. Neeraja, Comparative studies of spinel MnFe2O4 nanostructures: structural, morphological, optical, magnetic and catalytic properties. J. Nanosci. Nanotechnol. 15(12), 9732–9740 (2015). https://doi.org/10.1166/jnn.2015.10343
S.S. Fareed et al., Properties of SILAR deposited magnetite (Fe3O4) thin films: effect of bath temperatures. J Mater Sci: Mater Electron. 28(13), 9450–9455 (2017). https://doi.org/10.1007/s10854-017-6687-y
V.D. Mote, V.R. Huse, B.N. Dole, Synthesis and characterization of Cr doped ZnO nanocrystals. Sci. Res. (2012). https://doi.org/10.4236/wjcmp.2012.24035
S. Irfan, Y. Shen, S. Rizwan, H.-C. Wang, S.B. Khan, C.-W. Nan, Band-Gap engineering and enhanced photocatalytic activity of sm and mn doped BiFeO3 nanoparticles. J. Am. Ceram. Soc. 100(1), 31–40 (2017)
X. Chen, S. Shen, L. Guo, S.S. Mao, Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110(11), 6503–6570 (2010)
S.K. Tripathy, Refractive indices of semiconductors from energy gaps. Opt. Mater. 46, 240–246 (2015). https://doi.org/10.1016/j.optmat.2015.04.026
S.J. Iyengar, M. Joy, T. Maity, J. Chakraborty, R.K. Kotnala, S. Ghosh, Colloidal properties of water dispersible magnetite nanoparticles by photon correlation spectroscopy. RSC Adv. 6(17), 14393–14402 (2016). https://doi.org/10.1039/c5ra26488j
D. Chettri, K.J. Singh, M. Mathew, N.D. Gupta, A novel numerical approach for the calculation of refractive index of Wurtzite InxGa1–xN. Int. J. Mod. Phys. B 32(28), 1850315 (2018)
J. Chen, H.S. Hsu, Y.H. Huang, D.J. Huang, Spin-dependent optical charge transfer in magnetite from transmitting optical magnetic circular dichroism. Phys. Rev. B 98(8), 1–11 (2018). https://doi.org/10.1103/PhysRevB.98.085141
S. Yusub, P. Srinivasa Rao, D. Krishna Rao, Ionic conductivity, dielectric and optical properties of lithium lead borophosphate glasses combined with manganese ions. J. Alloys Compd. 663, 708–717 (2016). https://doi.org/10.1016/j.jallcom.2015.12.147
S.L.S. Rao, G. Ramadevudu, M. Shareefuddin, A. Hameed, M.N. Chary, M.L. Rao, Optical properties of alkaline earth borate glasses. Int. J. Eng. Sci. Technol. 4(4), 25–35 (2012). https://doi.org/10.4314/ijest.v4i4
S. Kawano, T. Yoshino, I. Katayama, Electrical conductivity of magnetite-bearing serpentinite during shear deformation. Geophys. Res. Lett. (2012). https://doi.org/10.1029/2012GL053652
Acknowledgements
This work would not have been possible without financial support from the PDUPT DRPM DIKTI research Grant on behalf of SN. The author would like to thank the PDUPT DRPM DIKTI for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sunaryono, Hidayat, M.F., Kholifah, M.N. et al. Study of Nanostructural, Electrical, and Optical Properties of Mn0.6Fe2.4O4–PEG/PVP/PVA Ferrogels for Optoelectronic Applications. J Inorg Organomet Polym 30, 4278–4288 (2020). https://doi.org/10.1007/s10904-020-01630-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01630-6