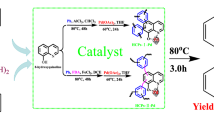
Abstract
Cu(I) nanoparticles were immobilized on modified poly(styrene-co-maleic anhydride) by Adenine with loading of 23.83% (w/w) of heterogeneous catalyst. The prepared nano catalyst was characterized by FTIR, 1H-NMR, SEM-EDAX, ICP-OES, TEM, BET and XRD. The catalytic activity of this novel heterogeneous Cu(I) nono particles was successfully examined in the regioselective synthesis of 1,4-disubstitued 1,2,3-triazoles via click reaction to give the corresponding products in satisfactory yield (78–87%). The heterogeneous catalyst was readily separated from reaction mixture and reused at least in four consecutive runs without loss of appreciable catalytic activity. Furthermore, a series of systematic computational studies was joint with experiment to answer some fundamental questions regarding the structure, stability, and nature of interactions in the novel inhomogeneous coordination nano catalyst.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Introduction of bio-related linkers to polymer backbone for the formation of polymer-supported catalysts is a topic which currently attracting much attention [1]. Amino acids, peptides, and nucleobases are readily accessible building blocks due to their abundance in nature. Since they contain a large number of oxygen and nitrogen atoms with different basicity, they show high tendency for being coordinated to various metals [2]. In particular, adenine which is one of the most common base present in nucleic acids, DNA and RNA is an interesting target. It is a rigid connector with different donor sites capable of coordinating with metallic ions [3]. Also, its versatility as a ligand has been extensively studied [4, 5].
Poly(styrene-co-maleic anhydride) (SMA) as a market purchasable co-polymer carrying reactive anhydride groups which can be modified using different nucleophiles for the purpose of its modification [6, 7] and preparation a wide range of heterogeneous catalysts [8].
In the art of organic synthesis, a revolutionary idea was independently introduced by Sharpless groups through the introduction of copper (I) catalysis termed as ‛Click Chemistry’ in 2001 [9]. This term was coined Sharpless for the regioselective formation of 1,4-disubstituted 1,2,3-triazoles [10]. As a matter of fact copper-catalyzed azide-alkyne Huisgen 1,3-dipolar cycloaddition [11] is called “Click Chemistry”.
Nowadays the application of heterogeneous catalysis is stated at the heart of the modern energy and chemical processes due to the high costs of the catalysts and to prevent the heavy metal contamination in pharmaceutically significant products for human use [12, 13]. Solid-supported heterogeneous catalysts have gained considerable interest in organic synthesis because of their unique properties like high efficiency, reusability, high stability, low toxicity and ease of handling [14].
Although nano catalysts [15, 16] are widely used in organic transformations due to high surface-to-volume ratio, the most common problems are their tedious filtration, centrifugation. They also often undergo agglomeration without the addition of stabilizers which thereby diminishing their activity. Therefore, efforts have been made to stabilize nanoparticles by immobilization on different solid supports [17, 18] such as polymers [14, 19]. Recently, much attention has also been paid on composite/hybrid nano catalysts with extraordinary performance on multidisciplinary approaches [20,21,22,23].
In the present paper, we wish to report the preparation and characterization of a novel polymer-supported nano copper catalyst and use it fruitfully as a heterogeneous catalyst in one the classiest organic name reaction so-called “Click Reaction” for regioselective synthesis of a series of 1,4-disubstitued -1,2,3-triazoles in satisfactory yields and in relatively short reaction times. The above-mentioned Cu(I) NPs immobilized onto polymer can be readily separated from reaction mixture by simple filtration due to its heterogeneous nature. Its potentiality for the consecutive recycling was also successfully examined, confirming its stabilization and its behavior as a potential and smart catalyst. The facile preparation of Cu(I)NPs immobilized onto a readily accessible modified polymer support together with the fact that no pre-activation step is required before the use of catalyst usage, can mentioned as the principle advantages of this immobilization strategy and technique. However, few number of disadvantages, which is common for most metal supported onto other polymers must be considered where applied. They are less or showing lack of enantioselectivity in asymmetric reactions relative to the homogeneous counterparts. They also relatively show more metal leaching during the courses of reactions.
The immobilization of copper (I) salts as NPs on polymers and their applications for the click synthesis of 1,2,3-triazoles between azides and alkynes have been reported [24, 25]. In addition, these catalysts like their homogeneous counterparts can be used under aqueous conditions to give the desired products in short reaction times and satisfactory yields [26, 27].
In continuation of our interest in the discovery of new heterogeneous catalysts [28, 29] to use in multi-component reactions [30, 31], Suzuki and Sonogashira cross-coupling reactions [32] and click reaction [1] and encouraged with our recent fascinating results from using modified SMA in click reaction and our joint work on experimental and computational [33, 34] such as Hantzsch reaction [35], herein, we wish to reveal our results on the synthesis of modified SMA using nucleobase adenine as a versatile coordinating agent along with in situ immobilization of Cu(I) nanoparticles. We also reveal our successful attempt to examine the activity of this new synthesized nanocatalyst in click reactions. Furthermore, the reusability and recyclability of this heterogeneous catalyst was examined. In addition, due to many numbers of site in adenine, DFT calculations were coupled with natural bond orbital (NBO) analysis and quantum theory of atoms in molecules (QTAIM) to determine how and why the metal ion coordinates with adenine group of Af_SMA and which nitrogen atom is preferred to coordinate with. The structure, stability, and nature of interactions in [Af_SMA-Cu]+1 coordination complexes were also evaluated quantitatively.
2 Experimental
2.1 Materials
N,N-Dimethylformamide (DMF) and triethylamine (TEA) were distilled and stored over 4 A° molecular sieve before use; all commercial solvents, chemicals and reagents were purchased from either Merck or Sigma-Aldrich with the as-purchased purity and used without further purification (except SMA which was obtained from KARABOND). The general formula of SMA (KARABOND SAM), used in this study, is [(C8H8)0.6 (C4H2O3)0.4]n with Anhydride/imide content = 40%, Mn (g/mol) = 86,666, Mw = 182,000, and Mw/Mn = 2.1.
2.2 Equipment
The 1H-NMR sppectra were recorded by a Bruker DPX, 300 MHz. FTIR spectra were recorded on FTIR Bruker Tensor 27 instrument, using KBr disks in the 500–4000 cm−1 region. The transmission electron and scanning electron micrographs for the catalyst surface were recorded using Philips CM30 TEM and Hitachi S4160 SEM instrument, respectively. Copper content was measured by inductively coupled plasma (ICP) on a Varian Vistapro analyzer.All products were known and identified by comparison of their physical and spectral data with those of authetic samples and found being indentical.
2.3 Preparation and Characterization of the Catalyst
2.3.1 Preparation of Adenine Functionalized SMA
The Adenine functionalized SMA (Af_SMA) was prepared according to the following protocol. SMA 1 (1.00 g), 9H-purin-6-amine (Adenine) 2 (2.16 g, 16 mmol) and dry DMF (15 ml) were placed in a 100 ml glass reactor then N2 gas was purged into the reactor and sealed. The reactor was located into a thermostatic oil bath and the mixture was stirred for 3.5 h at 35 °C until the reagents were dissolved in DMF. Then acetic anhydride (0.6 ml, 6 mmol), sodium acetate (0.33 g, 4 mmol), and Et3N (0.3 ml, 2 mmol) were added into the reactor via syringe (Scheme 1). The temperature was raised to 75 °C and reaction mixture was stirred for further 3.5 h. The whole mixture was then cooled to room temperature and added dropwise into 300 ml of vigorously stirring mixture of methanol:acetonitrile 1:1. The fiber-like precipitated polymer was collected by filtration, repeatedly washed with methanol:acetonitrile 1:1, and dried under reduced pressure at 70 °C to constant weight. For further purification, the Af_SMA polymer (3) was re-precipitated from DMF (Scheme 1). The amine content of Af_SMA was estimated by back titration using NaOH (0.2 N) [31]. In this regard, 10 ml of HCl (0.2 N) was added to 0.05 g of the Af_SMA and the resulted mixture was stirred for 30 min. The resulted Af_SMA was removed and washed consecutively with deionized water. After that, the excess amount of HCl was titrated with NaOH (0.2 N) in the presence of phenolphthalein as an indicator. Amine sites content of the synthesized Af_SMA found to be 9.90 mmol g−1.
2.3.2 Preparation of Polymer-Supported Catalyst
CuI (0.78 mg, 4.12 mmol) was dissolved in acetonitrile (2 ml) by ultrasonic irradiation and stirring, giving a transparent pale yellow solution. DMF (20 ml) was then added at room temperature to provide CuI nanoparticles (NPs) [36]. The Af_SMA polymer (1.000 g) was treated with CuI NPs mixture, and the reaction mixture was magnetically stirred at reflux temperature for 6 h under a nitrogen atmosphere. After filtration, the Af_SMA -CuI 4 was washed with acetonitrile and dried under vacuum at 60 °C overnight (Scheme 1). The filtrate was diluted to 50 ml with distilled water and analyzed by ICP-atomic emission spectrometry (AES). The copper content was determined being 23.83% w/w.
2.4 Synthesis of 1,2,3-Triazoles: General Procedure
In a round-bottom flask, the appropriative α-haloketones (1 mmol) or alkyl halide (1 mmol), alkyne (1 mmol) (entry 1–6), sodium azide (1.1 mmol), and 10 ml aqueous ethanol (1:1) were placed (Scheme 2). Next, Af_SMA-CuI catalyst 4 (0.01 g) was added, and the suspension was magnetically stirred under reflux for the required time according to Table 1. Since, the copper content in the catalyst was determined to be 23.83% (w/w), each gram of heterogeneous catalyst includes 1.25 mmol of copper. For 1 mmol of reactants, 0.01 mmol of catalyst is needed, which is equal to 0.01 g. The progress of the reaction was monitored by TLC (n-hexane: ethyl acetate; 7:3), and after the completion of the reaction, the resin was filtered off and washed with hot ethanol (5 ml). The recovered catalyst was washed with acetone, dried under reduced pressure at 70 °C for 3 h and stored for use in subsequent reactions. Crystalline products (7a-) were produced in the flask after a time. In some cases (7e–h), the filtrate was evaporated to dryness and the residue was washed with 10 ml of ether for better purifications. All the triazoles (7a–k) are known compounds, and their physical data were found to be identical with those of authentic samples [37]. Spectral (IR, 1H NMR and 13C NMR) data for selected compound is presented below.
3 Results and Discussion
3.1 Characterization of Af_SMA
Following the Lee and co-worker optimized reaction conditions [38], the chemical modification of SMA (1) was conducted in two steps (Scheme 1) [38]. Figure 1 represents FTIR spectrum of Af_SMA. The typical doublet of anhydride units which should be observed at 1860 and 1775 cm−1 in SMA [37] was omitted after the reaction of SMA with adenine and a new imide band around 1640 cm−1 is clearly observed as well as the expected band at 3300–3500 cm−1 for amino groups have been eliminated. The 1H NMR spectrum of Af_SMA d6-DMSO was obtained and presented in Supplementary materials (Figure S1). By its comparison with that of SMA [38], it was clearly determined that adenine has been effectively introduced to SMA. In addition, the aromatic protons for purine of residual adenine and also NH proton are observed as follows: [1.04 (m, 2H), 2.72 (s, 1H), 2.88 (s, 1H), 3.17 (s, 1H), 5.74 (m, 1H), 7.13 (1H, Ar–H), 7.9 (1H, Ar–H)].
The morphological characteristics of SMA and Af_SMA are determined by the SEM microphotographs analysis as shown in Fig. 2. After imidation, the diameter of the Af_SMA body was extended Cu(I) immobilized on the Af_SMA, thus the morphology of the SMA template is relatively changed (Fig. 2c, d). Energy dispersive spectroscopy analysis of X-rays (EDAX) data for this novel CuI-catalyst is depicted in Fig. 3. The EDAX data analysis also proves the perfect attachment of CuI to the surface of the polymer matrix. The FE-SEM and TEM micrographs of Af_SMA-CuI are depicted in Figs. 4 and 5, respectively. TEM image confirms that CuI nanoparticles were uniformly dispersed on the surface of Af_SMA analysis and indicated that the incorporated CuI nanoparticles had an average diameter of 80 nm.
The surface area of Af_SMA-CuI was determined by Brunauer-Emmett-Teller (BET) isotherm. The N2 adsorption–desorption isotherm and Barrett-Joyner-Halenda (BJH) pore size distribution plot of Af_SMA-CuI showed in Figure S2. The BET surface area, single point total pore volume and mean pore diameters are 32.45 m2 g−1, 0.1678 cm3 g−1 and 20.811 nm, respectively. The XRD patterns of polymeric structure (Figure S3) demonstrated a broad band in 2θ range of 25–35 that confirmed and assigned to the scattering amorphous polymer. As Figure S2 shows, the bands in 2θ of 35 and 38 can be attributed the incorporation of Cu (I) in the structure.
3.2 Computational Results
We also performed a series of computational studies based on density functional theory (DFT) calculations for presenting better indulgent of interaction between Cu as a metal with Af_SMA as a ligand. The main goal of the computational evaluations is to determine how and why the metal ion coordinates with adenine group of Af_SMA and more importantly which nitrogen atom is more favorite to coordinate with metal. To gain to the aforementioned goal, DFT calculations were coupled with natural bond orbital (NBO) analysis and quantum theory of atoms in molecules (QTAIM) was also employed. QTAIM analysis was performed on the calculated wave function of electron density using AIMALL program package [39]. All DFT and NBO calculations were performed using Gaussian 09 program package [40] at M06/6-311G(d, p) level of theory [41]. M06 is a hybrid meta-GGA exchange-correlation functional that was frequently used for systems including both transition metals and nonmetals and it is recommended in organometallic studies and non-covalent interactions [35]. LANL2DZ effective core potential [42] was used to describe the valence electron density in metal ion. Moreover, the effects of solvent (DMF) on the energetic features of coordination procedure have been investigated via polarized continuum model (PCM) [43]. It is worthy to mention that basis set super position error (BSSE) [44] was considered here using standard so-called counterpoise correction to modify the results. Furthermore, the vibrational zero point energy (ZPE) corrections were also considered in our calculations.
To make a reasonable balance between the accuracy and computational cost, we have used an effective model for Af_SMA including three repeating monomers with Adenine group attached to the middle monomer, the first and third monomer were saturated (Scheme 3). Based on the coordination site of metal ion, four possible complexes were constructed, namely complex1 to complex 4 (Scheme 3). As a first step, the structure of Af_SMA and four [Af_SMA-Cu]+1 coordination complexes were fully optimized and all stationary points were characterized as minima after verifying the absence of any virtual negative frequencies.
The coordination energy of Cu+1 with Af_SMA was calculated using the following equation;
where \({E_{{{[SMI - Cu]}^{+1}}}}\), \({E_{SMI}}\), and \({E_{C{u^{+1}}}}\)are the total energy of optimized structure of [Af_SMA-Cu]+1 coordination complexes, Af_SMA, and Cu+1, respectively. As mentioned before, the BSSE and vibrational ZPE corrections were considered during the calculations. The coordination energies for complexes 1–4 were calculated to be − 56.06, − 77.86, − 101.27, and − 29.16 kcal mol1, respectively.
The coordination energies for all complexes are negative, this implies that metal ion coordination with Af_SMA is possible with all four coordination sites. However, the coordination energy of complex 3 (− 101.27 kcal mol−1) is considerably more negative comparing with other three complexes. According to the calculated coordination energies, the complex 3 is more stable structure of [Af_SMA-Cu]+1.
To get a deep insight about this observation, a series of NBO calculations were done to extract the individual and total Wiberg bond index (WI) [45] of metal ion within different complexes. Table 1 indicates that the strongest Cu–N bond belongs to the complex 1 but the coordination energy calculations show that complex 3 is more stable one. This paradigm may be explained with this fact that the metal ion is engaged in more interactions in complex 3 and 2 rather than complex 1. For example the number of metal ion interactions with Wiberg bond index greater than 0.1 are 1, 2, 4, and 1 for complexes 1–4, respectively. The correlation between the total number of metal ion interactions and coordination energy would be perceived more quantitatively via total Wiberg bond index. The total Wiberg bond index is determined as sum of the individual Wiberg bond indices for all possible metal ion interactions.
The second order perturbation theory calculation is carried out to analyze the charge transfer phenomena during the coordination process for all possible interactions from NBO donors to metal ion as NBO acceptor. The energetic importance (delocalization stabilization energy) were also estimated. The results shown in Table 2. As Table 2 indicates, the charge transfer from NBO donors to metal ion leads to a considerable stabilization during the coordination reaction. For example, the charge transfer from the lone pairs of nitrogen atoms to empty orbitals of metal ion made the system to be more stable by 71.0, 60.7, 81.4, and 26.6 kcal mol−1 for complexes 1–4, respectively. The total stabilization energy (accounts for all possible interactions in which the metal ion roles as NBO acceptor) shows that the metal ion in complex 3 is engaged in more interactions that is consistent with the results obtained from both coordination energy calculations and total Wiberg bond indices. The NBO partial charges for metal ions are also calculated and shown in Table 3. It is expected that the partial charges of metal ion correlate with the magnitude of charge transfer from NBO donors to metal ion. Accordingly, the partial charge of metal ion in complex 3 (0.71 a.u) is smaller than the other three complexes.
The nature of interactions in [Af_SMA-Cu]+1 coordination complexes were evaluated using QTAIM calculations. Table 3 lists the QTAIM calculated parameters of electron density, \(\rho\), and its Laplacian, \({\nabla ^2}\rho\), at some selected critical bond points (BCPs). As Table 3 shows, all values of electron densities are equal or smaller than 0.10 and their Laplacian is positive. This set of QTAIM parameters (\(\rho \leqslant 0.10\) and \({\nabla ^2}\rho>0\)) is indicative of an unshared closed shell (mainly electrostatic) interaction [37].
The efficiency of this catalyst was examined in a sample click reaction for the preparation of triazoles derivatives as described below.
3.3 Optimization of the Reaction Conditions
For finding optimal reaction conditions, the reaction of 2-bromoacetophenone (1 mmol), sodium azide (1.1 mmol), and phenylacetylene (1 mmol) was selected as a model reaction and performed using various catalyst loading and in different of solvents namely, EtOH, H2O, and EtOH/H2O (1:1) (Table S1). The reaction times, using 0.03 g of catalyst, are 30, 19, and 36 min and corresponding yields are 80, 82, and 75% in EtOH,, and H2O respectively. As shown in Table S1, the best results were obtained for entry 2 (solvent EtOH/H2O). For the optimization of the amount of catalyst, the model reaction was performed in EtOH/H2O (1:1) (10 ml) using different amounts of the catalyst (Table 4).
The best result was achieved by carrying out the model reaction in the presence of 0–03 g of Af_SMA-CuI under reflux in EtOH/H2O (1:1) (Table 4, entry 2). By securing optimal reaction conditions, to establish the generality of this strategy and revealing substrate scope, various terminal alkynes and a haloketones or alkyl halides were reacted successfully in the presence the above-mentioned catalyst. (Table 5).
3.4 Recyclability of the Supported Catalyst
In a real heterogeneous catalysis, the recyclability of the catalyst is highly important, thus the supported catalyst should not leach into the reaction mixture. For investigation of this quality in the present system, the reaction of 2-bromoacetophenone, phenylacetylene, and sodium azide was selected again as model reaction (Table S2). Consistent activity of the catalyst was observed over 4 consecutive runs, without any need to reload the catalyst.
4 Conclusion
In conclusions, a novel Cu(I)NPs coordinated with adenine as a green ligand, immobilized onto the modified Af_SMA was successfully prepared, with appropriate CuI loading of 23.83% (w/w) which is relatively higher than most previously reports similar species. This adenine based Cu(I)NPs immobilized onto Af_SMA is a clean and green catalyst which showed being shelf-stable without any particular protection. Af_SMA-CuI was efficiently catalyzed the click reaction without showing significant leaching of copper and can be reused over four cycles without appreciable loss of its catalytic potency. Because of the existence of bio-related linkage in the polymer backbone, the polymer support is expected being biodegradable thus, classified under environmentally friendly polymers being used as support. It is worth noting that the resin did not suffer from extensive mechanical degradation after served catalytic runs. These benefits make this supported copper catalyst as an interesting and useful alternative to other Cu(I) heterogeneous catalytic systems and recommended, being tried in other Cu(I) catalyzed organic transformations.
References
F. Nemati, M.M. Heravi, A. Elhampour, RSC Adv. 5, 45775–45784 (2015)
I. Imaz, M. Rubio-Martínez, J. An, I. Sole-Font, N.L. Rosi, D. Maspoch, Chem. Commun 47, 7287–7302 (2011)
M.M. Heravi, H. Hamidi, V. Zadsirjan, Curr. Org. Synth. 11, 647–675 (2014)
I. Burneo, K.C. Stylianou, S. Rodriguez-Hermida, J. Juanhuix, X. Fontrodona, I. Imaz, D. Maspoch, Cryst. Growth Des. 15, 3182–3189 (2015)
J.P. García-Terán, O. Castillo, A. Luque, U. García-Couceiro, P. Román, L. Lezama, Inorg. Chem. 43, 4549–4551 (2004)
R. Chinchilla, D.J. Dodsworth, C. Nájera, J.M. Soriano, Tetrahedron Lett. 44, 463–466 (2003)
R. Chinchilla, D.J. Dodsworth, C. Nájera, J.M. Soriano, M. Yus, Arkivoc, 10 (2003) 41–47
J. Gil-Moltó, S. Karlström, C. Nájera, Tetrahedron, 61, 12168–12176 (2005)
H.C. Kolb, M. Finn, K.B. Sharpless, Angew. Chem. Int. Ed. 40, 2004–2021 (2001)
H.C. Kolb, K.B. Sharpless, Drug Discov Today 8, 1128–1137 (2003)
M.M. Heravi, M. Tamimi, H. Yahyavi, T. Hosseinnejad, Curr. Org. Chem. 20, 1591–1647 (2016)
P. Munnik, P.E. de Jongh, K.P. de Jong, Chem. Rev. 115, 6687–6718 (2015)
R. Nasir Baig, R.S. Varma, ACS Sustain. Chem. Eng. 2, 2155–2158 (2014)
R.N. Baig, R.S. Varma, Green Chem. 14, 625–632 (2012)
M. Honda, T. Goto, T. Owashi, A.G. Rozhin, S. Yamaguchi, T. Ito, S.A. Kulinich, Phys. Chem. Chem. Phys. 18, 23628–23637 (2016)
K.R. Reddy, V.G. Gomes, M. Hassan, Mater. Res. Express 1, 015012 (2014)
K.R. Reddy, B.C. Sin, C.H. Yoo, W. Park, K.S. Ryu, J.-S. Lee, D. Sohn, Y. Lee, Scripta Mater. 58, 1010–1013 (2008)
A.M. Showkat, Y.-P. Zhang, M.S. Kim, A.I. Gopalan, K.R. Reddy, K. Lee, Bull.-Korean Chem. Soc. 28 1985 (2007)
K.R. Reddy, K.P. Lee, A.I. Gopalan, Colloids Surf. A 320, 49–56 (2008)
Y. Kofuji, S. Ohkita, Y. Shiraishi, H. Sakamoto, S. Tanaka, S. Ichikawa, T. Hirai, ACS Catal. 6, 7021–7029 (2016)
K.R. Reddy, K. Nakata, T. Ochiai, T. Murakami, D.A. Tryk, A. Fujishima, J. Nanosci. Nanotechnol. 11, 3692–3695 (2011)
K.R. Reddy, K. Karthik, S.B. Prasad, S.K. Soni, H.M. Jeong, A.V. Raghu, Polyhedron, 120, 169–174 (2016)
K.R. Reddy, M. Hassan, V.G. Gomes, Appl. Catal. A 489, 1–16 (2015)
C. Girard, E. Önen, M. Aufort, S. Beauvière, E. Samson, J. Herscovici, Org. Lett. 8, 1689–1692 (2006)
A. Coelho, P. Diz, O. Caamano, E. Sotelo, Adv. Synth. Catal. 352, 1179–1192 (2010)
I.S. Park, M.S. Kwon, Y. Kim, J.S. Lee, J. Park, Org. Lett. 10, 497–500 (2008)
J. Albadi, M. Keshavarz, F. Shirini, M. Vafaie-nezhad, Catal. Commun. 27, 17–20 (2012)
M.M. Heravi, K. Bakhtiari, Z. Daroоgheha, F.F. Bamoharram, J. Mol. Catal. A 273, 99–101 (2007)
M.M. Heravi, K. Bakhtiari, Z. Daroogheha, F.F. Bamoharram, Catal. Commun. 8, 1991–1994 (2007)
M.M. Heravi, E. Hashemi, Y.S. Beheshtiha, K. Kamjou, M. Toolabi, N. Hosseintash, J. Mol. Catal. A 392, 173–180 (2014)
M.M. Heravi, E. Hashemi, F. Azimian, J. Iran. Chem. Soc. 12, 647–653 (2015)
M.M. Heravi, E. Hashemi, Y.S. Beheshtiha, S. Ahmadi, T. Hosseinnejad, J. Mol. Catal. A 394, 74–82 (2014)
T. Hossiennejad, M. Daraie, M.M. Heravi, N.N. Tajoddin, J. Inorg. Organomet. Polym. Mater. (2017). https://doi.org/10.1007/s10904-017-0530-z
T. Baie Lashaki, H.A. Oskooie, T. Hosseinnejad, M.M. Heravi, J. Coord. Chem. (2017). https://doi.org/10.1080/00958972.2017.1321742
M.M. Heravi, T. Hosseinnejad, N. Nazari, Can. J. Chem. 95 530–536 (2017)
R.J. Kalbasi, M. Kolahdoozan, M. Rezaei, J. Ind. Eng. Chem. 18, 909–918 (2012)
E. Hashemi, Y.S. Beheshtiha, S. Ahmadi, M.M. Heravi, Transit. Met. Chem. 39, 593–601 (2014)
S.S. Lee, T.O. Ahn, J. Appl. Polym. Sci. 71, 1187–1196 (1999)
T.A. Keith, T. Gristmill, AIMAll (Version 16.10. 31) (Software, Overland Park, 2016)
M. Frisch, G. Trucks, H.B. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. Petersson, Gaussian 09 (Gaussian Inc., Wallingford, 2009), p 200
Y. Zhao, D.G. Truhlar, Theoretical chemistry accounts: theory, computation, and modeling. Theor. Chim. Acta 120, 215–241 (2008)
L.E. Roy, P.J. Hay, R.L. Martin, J. Chem. Theory Comput. 4, 1029–1031 (2008)
V. Barone, M. Cossi, J. Phys. Chem. A 102, 1995–2001 (1998)
S.F. Boys, F.D. Bernardi, Mol. Phys. 19, 553–566 (1970)
K.B. Wiberg, Tetrahedron 24, 1083–1096 (1968)
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heravi, M.M., Mahdizade, S.J., Esfandiari, M. et al. Experimental and Computational Studies on Catalytic Activity of Novel Adenine-Based Nano Cu(I) Polymers in Regioselective Synthesis of 1,4-Disubstituted 1,2,3-Triazoles. J Inorg Organomet Polym 28, 767–776 (2018). https://doi.org/10.1007/s10904-017-0727-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0727-1