Abstract
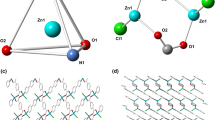
Here we report the mechanochemical synthesis of Zn(ADB)n coordination polymer (ADB: azobenzene 4, 4′-dicarboxilic acid) in good yield, which provides advanced over conventional route as it is simple, faster, cost-effective and reproducible. Both coordination polymers were characterized by X-ray powder diffraction (PXRD), field emission scanning electron microscopy, energy dispersive X-ray spectroscopy, and simultaneous thermal analysis. PXRD studies showed that both materials exhibited the same pattern, proving the presence of the crystalline coordination polymer core. FESEM micrograph images showed plate shape microstructures with a length of 10 µm, and a thickness in range from 14 to 21 µm, which suggested that synthetic routes affect the coordination polymer morphology. The resulting coordination polymers have low porosity with surface areas around from 1.92 to 1.25 m2 g− 1 and pore sizes in range from 0.36 to 0.61 nm for mechanochemical and room temperature stirring routes, respectively. In a preliminary fluorescent sensing study, the coordination polymer soaked with different volatile organic compounds showed noticeable shifts of the emission band in comparison with the same materials free solvent.
Graphical Abstract







Similar content being viewed by others
References
A. Corma, H. García, F.X. Llabrési Xamena, Chem. Rev. 110, 4606 (2010)
L. Ma, C. Abney, W. Lín, Chem. Soc. Rev. 38, 1248 (2009)
J. Gascon, A. Corma, F. Kapteijnand, F.S. Llabrési Xamena, ACS Catal. 4, 361 (2013)
J. Canivet, D. Farrusseng, Application of metal–organic frameworks in fine J. chemical synthesis, K. Wilson, A.F. Lee, Heterogeneous Catalysts for Clean Technology: Spectroscopy, Design, and Monitoring, (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013)
M.C. Muñoz, J.A. Real, M.A. Halcrow, Spin-Crossover Materials: Properties and Applications, (Wiley, Chichester, 2013)
D.-F. Weng, Z.-M. Wang, S. Gao, Chem. Soc. Rev. 40, 3157 (2011)
D.R. Talham, M.W. Meisel, Chem. Soc. Rev. 40, 3356 (2011)
G. Givaja, P. Amo-Ochoa, C.J. Gómez-García, F. Zamora, Chem. Soc. Rev. 41, 115 (2012)
J. Heine, K. Müller-Buschbaum, Chem. Soc. Rev. 42, 9232 (2013)
K. Binnemans, Chem. Rev. 109, 4283 (2009)
M.D. Allendorf, C.A. Bauer, R.K. Bhakta, R.J.T. Houk, Chem. Soc. Rev. 38, 3013 (2009)
L.J. Murray, M. Dincă, J.R. Long, J. Mater. Chem. 21, 17259 (2011)
T.A. Makal, J.-R. Li, W. Lu, H.-C. Zhou, Chem. Soc. Rev. 41, 7761 (2012)
M.P. Suh, H.J. Park, T.K. Prasad, D.-W. Lim, Chem. Rev. 112, 782 (2012)
Y. He, W. Zhou, G. Quian, B. Chen, Chem. Soc. Rev. 43, 5657 (2014)
B. Van de Voorde, J. Denayer, D. De Vos, Chem. Soc. Rev. 43, 5766 (2014)
P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J.F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, Nat. Mater. 9, 172 (2010)
J. Della Rocca, D. Liu, W. Lin, Acc. Chem. Res. 44, 957 (2011)
P. Horcajada, R. Gref, T. Baati, P.K. Allan, G. Maurin, P. Couvreur, G. Férey, R.E. Morris, C. Serre, Chem. Rev. 112, 1232 (2011)
C.-Y. Sun, C. Qin, X.-L. Wangand, Z.-M. Su, Expert Opin. Drug Deliv. 10, 89 (2013)
V. Stavila, A.A. Talin, M.D. Allendorf. Chem. Soc. Rev. 43, 6011 (2014)
M. Du, Z.H. Zhang, L.F. Tang, X.G. Wang, X.J. Zhao, S.R. Batten, Chem. Eur. J. 13, 2578 (2007)
C.Y. Su, A.M. Goforth, M.D. Smith, P.J. Pellechia, H.C.Z. Loye, J. Am. Chem. Soc. 126, 3576 (2004)
O.M. Yaghi, M. O’Keeffe, N.W. Ockwig, H.K. Chae, M. Eddaoudi, J. Kim, Nature 423, 705 (2003)
Z.G. Li, G.H. Wang, H.Q. Jia, N.H. Hu, J.W. Xu, CrystEngComm 9, 882 (2007)
B. Chen, S. Xiang, G. Qian, Acc. Chem. Res. 43, 1115 (2010)
Z. Hu, B.J. Deibert, J. Li, Chem. Soc. Rev. 43, 5815 (2014)
L. Zhang, Z. Kang, X. Xin, D. Sun, CrysEngComm 18, 193 (2016)
Y. Cui, Y. Yue, G. Quian, B. Chen, Chem. Rev. 112, 1126 (2012)
H. Sakamoto, R. Matsuda, S. Kitagawa. Dalton Trans. 41, 3956 (2012)
A. Pichon, A. Lazuen-Garay, S.L. James, CrysEngComm 8, 211 (2006)
P. Horcajada, R. Gref, T. Baati, P.K. Allan, G. Maurin, P. Couvreur, G. Férey, E.R. Morris, C. Serre, Chem. Rev. 112, 1232 (2012)
M. Schlesinger, S. Schulze, M. Hietschold, M. Mehring, Microporous Mesoporous Mater. 132, 121 (2010)
M. Klimakow, P. Klobes, A.F. Thünemann, K. Rademann, F. Emmerling, Chem. Mater. 22, 5216 (2010)
B. Liu, Q. Xu, Acta Cryst. E65, m509 (2009)
F. Fu, D.-S. Li, X.-G. Yang, C.-Q. Zhang, Y.-P. Wu, J. Zhao, E.-B. Wang, Inorg. Chem. Commun. 12, 657 (2009)
T. Friščić, Metal-Organic Framework, 1st ed. (Wiley, Hoboken, 2010)
J. Hafizovic, M. Bjorgen, U. Olsbye, P.D.C. Dietzel, S. Bordiga, C. Prestipino, C. Lamberti, K.P. Lillerud, J. Am. Chem. Soc. 129, 3612 (2007)
A. Schaate, S. Dühnen, G. Platz, S. Lilienthal, A.M. Schneider, P. Behrens, Eur. J. Inorg. Chem. 5, 790 (2012)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, Pure Appl. Chem. 57, 603 (1985)
J. Lincke, D. Lässiga, J. Moellmer, C. Reichenbach, A. Puls, A. Moeller, R. Gläser, G. Kalies, R. Staudt, H. Krautscheid, Microporous Mesoporous Mater. 142, 62 (2011)
M. Zhang, G. Feng, Z. Song, Y.-P. Zhou, H.-Y. Chao, D. Yuan, T.T.Y. Tan, Z. Guo, Z. Hu, B.Z. Tang, B. Liu, D. Zhao, J. Am. Chem. Soc. 136, 7241 (2014)
Acknowledgements
The authors thank the CONACYT for a Research Grant (240011). D. C. C. thanks for scholarship from CONACYT.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chan-Navarro, R., Corpus-Coronado, D., Muñoz-Flores, B.M. et al. Luminescent Sensing of Volatile Organic Compounds Using a Zn-based Coordination Polymer with Tunable Morphology. J Inorg Organomet Polym 27, 467–473 (2017). https://doi.org/10.1007/s10904-016-0488-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-016-0488-2