Abstract
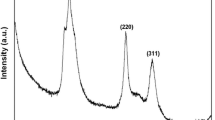
CdSe quantum dots (QDs) having size 3–5 nm have been synthesized by chemical co-precipitation method in mixed amorphous and cubic phase. These QDs have been characterized by using X-ray diffraction (XRD), high resolution transmission electron microscopy (HRTEM) and Fourier transform infrared (FTIR) spectrometry. Cubic phase is confirmed by XRD and amorphous phase have been found using differential scanning calorimetry (DSC). The thermal analysis carried out by using DSC at different heating rates shows endothermic and exothermic peaks at different temperatures corresponding to their glass transition and different crystalline phases. The cubic phase of the crystalline CdSe obtained at 261 °C in the DSC scan of as prepared sample start transforming to stable hexagonal phase of CdSe corresponding to second crystallization peak 317 °C in DSC thermogram. Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) have also been used to determine the crystallization temperature (257 °C) and weight loss in the temperature range 220–267 °C. Activation energies corresponding to two exothermic peaks (cubic and hexagonal phase) have been determined by employing different theoretical models. Lower activation energy of the hexagonal phase corresponding to second exothermic peak obtained at higher temperature in the DSC thermogram shows the higher thermal stability of this phase and could be employed for preparing a material to improve the performance of memory devices, solar cells etc.










Similar content being viewed by others
References
Y. Wu, C. Wadia, W. Ma, B. Sadtler, A.P. Alivisatos, Nano Lett. 8, 2551–2555 (2008)
I. Gur, N.A. Fromer, M.L. Geier, A.P. Alivisatos, Science 310, 462–465 (2005)
R. Gangadharan, V. Jayalakshmi, J. Kalaiselvi, S. Mohan, R. Murugan, B. Palanivel, J. Alloys Comp. 359, 22–26 (2003)
A. Kanti, P. Kumbhakar, Res. Phys. 2, 150–155 (2012)
N.A. Hosiny, A. Badawai, M.A.A. Moussa, R.E. Agmy, S. Abdallah, Int. J. Nanoparticles. 5, 258–266 (2012)
S.R. Dhage, H.A. Colorado, H.T. Hahn, Mater. Res. 16, 504–507 (2013)
G.A.D. Silva, D.M. Triches, E.A. Sanches, K.D. Machado, C.M. Poffo, J.C.D. Lima, S.M.D. Souza, J. Mole. Struct. 1074, 511–515 (2014)
R.J. Bandaranayake, G.W. Wen, J.Y. Lin, H.X. Jiang, C.M. Sorensen, Appl. Phys. Lett. 67, 831–833 (1995)
H.E. Kissinger, Anal. Chem. 29, 1702–1706 (1957)
J.A. Augis, J.E. Bennett, J. Therm. Anal. Calor. 13, 283–292 (1978)
T. Ozawa, Polymer 12, 150–158 (1971)
M. Verma, D. Patidar, K.B. Sharma, N.S. Saxena, J. Nanoelectron. Optoelectron. 10, 320–326 (2015)
M.F. Kotkata, A.E. Masoud, M.B. Mohamed, M.A. Mahmoud, Physica E 41, 640–645 (2009)
T.S. Shyju, S. Anandhi, R. Indirajith, R. Gopalakrishnan, J. Crys. Growth. 337, 38–45 (2011)
M. Avarmi, J. Chem. Phys. 7, 1103–1112 (1939)
M. Avarmi, J. Chem. Phys. 8, 12–224 (1940)
Acknowledgments
Authors gratefully acknowledge the financial grant received from UGC, New Delhi (India) in the form of Emeritus Fellowship to Prof. N.S. Saxena and BSR fellowship (JRF) to Mr. M. Verma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, M., Patidar, D., Sharma, K.B. et al. Phase Transformations and Thermal Stability of CdSe Quantum Dots: Cubic to Hexagonal. J Inorg Organomet Polym 26, 75–80 (2016). https://doi.org/10.1007/s10904-015-0297-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0297-z