Abstract
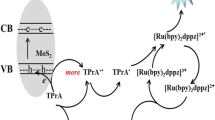
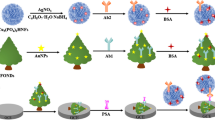
A sensitivity-enhanced electro chemiluminescence immunoassay (ECLIA) was fabricated by covalently immobilizing a monoclonal prostate specific antigen (PSA) antibody (anti-PSA, Ab2) and a luminophore (luminol) on dendrimer-encapsulated gold nanoparticles (Den/AuNPs) as electrochemiluminescence labels. The primary antibody was immobilized on Fe3O4@SiO2 NP support, and the antibody-loaded Fe3O4@SiO2 NP was placed onto an indium tin oxide working electrode in a home made electrochemiluminescence (ECL) cell. PSA and Ab2/Luminol/Den/AuNP was successively injected into the cell, and conjugated to form a sandwich-type immunocomplex. Under optimized experimental conditions, the proposed ECLIA provided a linear response range from 0.001 to 100.0 ng/mL with a low detection limit of 0.3 pg/mL. The PSA assay results in clinical serum samples were in good agreement with the commercially available electro chemiluminescence assay. The ECL immunosensor has the advantages of high sensitivity, specificity and stability and may be a promising technique for tumor marker detection.









Similar content being viewed by others
References
S.J. Xu, Y. Liu, T.H. Wang, J.H. Li, Anal. Chem. 83, 3817 (2011)
J.L. Munro, V.A. Boon, J. Agric. Food Chem. 58, 1429 (2010)
Y.Q. Shang, R. Mernaugh, X.Q. Zeng, Anal. Chem. 84, 8164 (2012)
S.J. Goldsmith, Semin. Nucl. Med. 5, 125 (1975)
A.M. Teppo, C.P.J. Maury, Clin. Chem. 33, 2024 (1987)
L.C. Zhou, F. Ding, H. Chen, W. Ding, W.H. Zhang, S.Y. Chou, Anal. Chem. 84, 4489 (2012)
T. Kreisig, R. Hoffmann, T. Zuchner, Anal. Chem. 83, 4281 (2011)
D. Kim, K. Karns, S.Q. Tia, M. He, A.E. Herr, Anal. Chem. 84, 2533 (2012)
O. Trenchevska, E. Kamcheva, D. Nedelkov, J. Proteome Res. 9, 5969 (2010)
G.S. Lai, F. Yan, H.X. Ju, Anal. Chem. 81, 9730 (2009)
S.R. Oppenheimer, D.M. Mi, M.E. Sanders, R.M. Caprioli, J. Proteome Res. 9, 2182 (2010)
S.H. Yuk, K.S. Oh, S.H. Cho, B.S. Lee, S.Y. Kim, B.K. Kwak, K. Kim, I.C. Kwon, Biomacromolecules 12, 2335 (2011)
C. Zong, J. Wu, C. Wang, H.X. Ju, F. Yan, Anal. Chem. 84, 2410 (2012)
X.Q. Liu, R. Freeman, E. Golub, I. Willner, ACS Nano 5, 7648 (2011)
W.W. Tu, W.J. Wang, J.P. Lei, S.Y. Deng, H.X. Ju, Chem. Commun. 48, 6535 (2012)
S.L. Zhao, J.W. Liu, Y. Huang, Y.M. Liu, Chem. Commun. 48, 699 (2012)
H.L. Qi, C. Ling, Q.Y. Ma, Q. Gao, C.X. Zhang, Analyst 137, 393 (2012)
S.G. Ge, L. Ge, M. Yan, X.R. Song, J.H. Yu, J.D. Huang, Chem. Commun. 48, 9397 (2012)
S.G. Ge, F. Yu, L. Ge, M. Yan, J.H. Yu, D.R. Chen, Analyst 137, 4727 (2012)
D. J. Zang, L. Ge, M. Yan, X. R. Song, J. H. Yu, Chem. Commun. 48, 4683 (2012)
M. Yan, L. Ge, W.Q. Gao, J.H. Yu, X.R. Song, S.G. Ge, Z.Y. Jia, C.C. Chu, Adv. Funct. Mater. 22, 3899 (2012)
L. Ge, J.X. Yan, X.R. Song, M. Yan, S.G. Ge, J.H. Yu, Biomaterials 33, 1024 (2012)
S. W. Wang, W. J. Dai, L. Ge, M. Yan, J. H. Yu, X. R. Song, S. G. Ge and J. D. Huang. Chem. Commun. 48, 9971 (2012)
C.G. Shi, X. Shan, Z.Q. Pan, J.J. Xu, C. Lu, N. Bao, H.Y. Gu, Anal. Chem. 84, 3033 (2012)
H. Jiang, X.M. Wang, Anal. Chem. 84, 6986 (2012)
D.P. Huang, Y.Y. Qi, X.T. Bai, L.J. Shi, H. Jia, D.J. Zhang, L.Q. Zheng, ACS Appl. Mater. Interfaces 4, 4665 (2012)
X.T. Bai, L.Q. Zheng, Cryst. Growth Des. 10, 4701 (2010)
D.P. Huang, X.T. Bai, L.Q. Zheng, J. Phys. Chem. C 115, 14641 (2011)
M.A. Rahman, H.B. Noh, Y.B. Shim, Anal. Chem. 80, 8020 (2008)
L.H. Zhang, B.F. Liu, S.J. Dong, J. Phys. Chem. B 111, 10448 (2007)
M.J. Li, Z.F. Chen, V.W.W. Yam, Y.B. Zu, ACS Nano 2, 905 (2008)
X.M. Zhou, D. Xing, D.B. Zhu, L. Jia, Anal. Chem. 81, 255 (2009)
R.X. Duan, X.M. Zhou, D. Xing, Anal. Chem. 82, 3099 (2010)
G.F. Jie, L. Wang, J.X. Yuan, S.S. Zhang, Anal. Chem. 83, 3873 (2011)
K.L. Metera, K.D. Hänni, G.N. Zhou, M.K. Nayak, H.S. Bazzi, D. Juncker, H.F. Sleiman, ACS Macro. Lett. 1, 954 (2012)
A. Dong, S. Lan, J.F. Huang, T. Wang, T.Y. Zhao, L.H. Xiao, W.W. Wang, X. Zheng, F.Q. Liu, G. Gao, Y.X. Chen, ACS Appl. Mater. Interfaces 3, 4228 (2011)
M.F. Shao, F.Y. Ning, J.W. Zhao, M. Wei, D.G. Evans, X. Duan, J. Am. Chem. Soc. 134, 1071 (2012)
Y.H. Won, H.S. Jang, S.M. Kim, E. Stach, M. Ganesana, S. Andreescu, L.A. Stanciu, Langmuir 26, 4320 (2010)
J. Su, M.H. Cao, L. Ren, C.W. Hu, J. Phys. Chem. C115, 14469 (2011)
Y.J. Song, K.G. Qu, C. Xu, J.S. Ren, X.G. Qu, Chem. Commun. 46, 6572 (2010)
B.J. Jeong, R. Akter, O.H. Han, C.K. Rhee, M.A. Rahman, Anal. Chem. 85, 1784 (2013)
M.A. Rahman, H.B. Noh, Y.B. Shim, Anal. Chem. 80, 8020 (2008)
M.J. Ruedas-Rama, E.A.H. Hall, Anal. Chem. 80, 8260 (2008)
Acknowledgments
This work was financially supported by the Natural Science Research Foundation of China (21207048), Major State Basic Research Development Program of China (973 Program) (no. 2010CB933504), Natural Science Foundation of Shandong Province, China (ZR2011BQ019), Youth Science and Technology Jinan star program (20110102) and State Key Laboratory of Environmental Chemistry and Ecotoxicology Research Center for Eco-Environmental Sciences Chinese Academy of Sciences (KF2011-03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, S., Yu, J., Jiao, X. et al. Ultrasensitive Electrochemiluminescence Immunoassay for Protein Specific Detection Based on Dendrimer-Encapsulated Gold Nanoparticles Labels. J Inorg Organomet Polym 23, 1113–1121 (2013). https://doi.org/10.1007/s10904-013-9895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-013-9895-9