Abstract
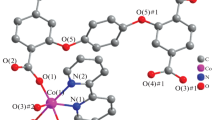
Two novel three-dimensional coordination polymers, formulated as {[M2(μ-C4H4O6)2(H2O)] · 3H2O}∞ (M = Mn for 1 and Cd for 2), have been synthesized under hydrothermal reaction condition. Both complexes were characterized by elemental analysis and IR spectroscopy. Their molecular and crystal structures were determined by X-ray crystal structure analysis and their thermal stability by TGA-DTA methods. Compound 1 crystallizes in the monoclinic space group, P21, while compound 2 crystallizes the orthorhombic space group, P212121. The structures are self-assembled from bifunctional tartrate and water molecules. Tartrate ligands in 1 and 2 contribute to both covalent and hydrogen bonds. Polymeric chains of 1 and 2 are composed of M(II) ions bridged by tartrate ions in O,O′ fashion. The asymmetric units of coordination polymers contain two metal centers having different coordination environments.






Similar content being viewed by others
References
X. Lin, C. Yun-Xia, Z. Ji-Min, Chinese J. Struct. Chem. 25, 1250 (2006)
Y.H. Wen, J.K. Cheng, Y.L. Feng, J. Zhang, Z.J. Li, Y.G. Yao, Inorg. Chim. Acta 358, 3347 (2005)
H.D. Yin, F.H. Li, C.H. Wang, Inorg. Chim. Acta 360, 2797 (2007)
H. Yin, S.X. Liu, Polyhedron 26, 3103 (2007)
A. Thirumurugan, S. Natarajan, Eur. J. Inorg. Chem. 762 (2004)
J.C. Yao, W. Huang, B. Li, S. Gou, Y. Xu, Inorg. Chem. Commun. 5, 711 (2002)
X.M. Zhang, M.L. Tong, M.L. Gong, X.M. Chen, Eur. J. Inorg. Chem. 138 (2003)
H. Li, C.E. Davis, F.L. Croy, D.G. Kelley, O.M. Yaghi, J. Am. Chem. Soc. 120, 2186 (1998)
L.L. Johnston, J.H. Nettleman, M.A. Braverman, L.K. Sposato, R.M. Supkowski, R.L. LaDuca, Polyhedron 29, 303 (2010)
Y.-Q. Zheng, X.-Y. Han, H.-L. Zhu, Polyhedron 29, 911 (2010)
M. Tabatabaee, V. Razavimahmoudabadi, B.-M. Kukovec, M. Ghassemzadeh, B. Neumüller, J. Inorg. Organomet. Polym. doi:10.1007/s10904-011-9462-1
C. Xie, B. Zhang, X. Wang, R. Wang, G. Shen, D. Shen, J. Chem. Crystallogr. 37, 25 (2007)
M. Tabatabaee, M.A. Sharif, F. Vakili, S. Saheli, J. Rare. Earth. 27, 356 (2009)
F. Semerci, O.Z. Yeşilel, E. Şahin, J. Inorg. Organomet. Polym. 20, 334 (2010)
Y.-Q. Zheng, J.-L. Lin, Z.-P. Kong, Inorg. Chem. 43, 2590 (2004)
Y.-Q. Zheng, D.-Y. Cheng, J.-L. Lin, Z.-F. Li, X.-W. Wang, Eur. J. Inorg. Chem. 17, 4453 (2008)
Y.-Q. Zheng, H.-Z. Xie, J. Solid State Chem. 177, 1352 (2004)
M.M. Petit-Ramel, C.M. Blance, J. Inorg. Nucl. Chem. 34, 1241 (1972)
V. Baliukiené, A. Surviliené, A. Survila, Chemija 12, 216 (2001)
S.C. Manna, E. Zangrando, J. Ribasc, N.R. Chaudhuri, Polyhedron 25, 1779 (2006)
H. Guo, Q. Liu, L. Yang, W. Weng, Q. Wanga, C. Zheng, Inorg. Chem. Commun. 11, 859 (2008)
F. Jian, P. Zhao, Q. Wang, J. Coor. Chem. 58, 1133 (2005)
M. Athar, G. Li, Z. Shi, Y. Chen, S. Feng, Solid State Sci. 10, 1853 (2008)
Z. Otwinowski, W. Minor, Methods Enzymol. 276, 307 (1997)
R. Hooft, COLLECT (Nonius BV, Delft, The Netherlands, 1998)
A. Altomare, M. Cascarano, C. Giacovazzo, A. Guagliardi, SIR92. J. Appl. Cryst. 26, 343 (1993)
P.T. Beurskens, G. Admiraal, G. Beurskens, W.P. Bosman, R. de Gelder, R. Israel, J.M.M. Smits, The DIRDIF-94 program system, Technical Report of the Crystallography Laboratory, University of Nijmegen, The Netherlands
G.M. Sheldrick, Acta Cryst. A64, 112 (2008)
L.J. Farrugia, J. Appl. Cryst. 30, 565 (1997)
C. Ge, Z. Zhao, G. Han, X. Zhang, Acta Cryst. E64, m36 (2008)
R.G. Xiong, C.M. Liu, J.L. Zuo, H.Z. Li, X.Z. You, H.K. Fun, K. Sivakumar, Polyhedron 16, 2315 (1997)
M. Tabatabaee, B.-M. Kukovec, M. Kazeroonizadeh, Polyhedron 30, 1114 (2011)
H. Aghabozorg, E. Sadr-khanlou, A. Shokrollahi, M. Ghaedi, M. Shamsipur, J. Iran. Chem. Soc. 6, 55 (2009)
Acknowledgments
This research was supported by Yazd Branch, Islamic Azad University, Yazd, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabatabaee, M., Gholamighavamabad, A., Khabiri, E. et al. Two Tartrate-Pillared Coordination Polymers: Hydrothermal Preparation, Crystal Structures, Spectroscopic and Thermal Analyses of {[M2(μ-C4H4O6)2(H2O)] · 3H2O}∞ (M = Mn, Cd). J Inorg Organomet Polym 21, 627–633 (2011). https://doi.org/10.1007/s10904-011-9495-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-011-9495-5