Abstract
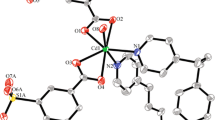
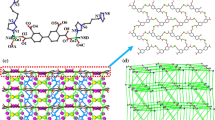
Two new coordination polymers [Mn(oba)(1,10-phen)] n (1) and [Zn(oba)(Pbim)2] n (2) [H2oba = 2-(3-carboxyphenoxy)benzoic acid, 1,10-phen = 1,10-phenanthro line and pbim = 2-(2-Pyridyl)benzimidazole] have been synthesized and structurally characterized by elemental analysis, IR and X-ray diffraction. Single-crystal X-ray analyses revealed that 2,3′-oba ligand act as a bridging ligand, exhibiting three coordination modes to link metal ion: bis-monodentate, bidentate chelating and monodentate modes. Compound 1 and 2 all demonstrate a 1D chain. There exist intermolecular hydrogen bonds which further connect the 1D structure into 2D supramolecular structure in compound 1 and 2. In addition, the luminescent property for compound 2 is also investigated in the solid state at room temperature.



Similar content being viewed by others
References
M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’Keeffe, O.M. Yahi, Science 295, 469 (2002)
S.S. Kaye, A. Dailly, O.M. Yaghi, J.R. Long, J. Am. Chem. Soc. 129, 14176 (2007)
L. Pan, H. Liu, X. Lei, X. Huang, D. Olson, N. Turro, J. Li, Angew. Chem. Int. Ed. 42, 542 (2003)
B. Moulton, M.J. Zaworotko, Chem. Rev. 101, 1629 (2001)
J.B. Lin, J.P. Zhang, X.M. Chen, J. Am. Chem. Soc. 132, 6654 (2010)
C.C. Ji, L.F. Huang, J. Li, H.G. Zheng, Y.Z. Li, Z.J. Guo, Dalton Trans. 39, 8240 (2010)
J. Li, W. Bi, W. Ki, X. Huang, S. Reddy, J. Am. Chem. Soc. 129, 14140 (2007)
S. Kitagawa, R. Kitaura, S. Noro, Angew. Chem. Int. Ed. 43, 2334 (2004)
S.R. Batten, R. Robson, Angew. Chem. Int. Ed. 37, 1460 (1998)
L.F. Chen, Z.J. Li, Y.Y. Qin, J.K. Chen, Y.G. Yao, J. Mol. Struct. 892, 278 (2008)
F. Guo, X.L. Zhang, B.Y. Zhu, J.C. Qiu, J. Inorg. Organomet. Polym. 20, 38 (2010)
G.W. Yang, Q.Y. Li, Y. Zhou, G.Q. Gu, Y.S. Ma, R.X. Yuan, Inorg. Chim. Acta 362, 1234 (2009)
Y.Q. Wei, Y.F. Yu, R.J. Sa, Q.H. Li, K.C. Wu, Cryst. Eng. Commun. 11, 1054 (2009)
B.J. Holliday, C.A. Mirkin, Angew. Chem. Int. Ed. 40, 2022 (2001)
F. Guo, B.Y. Zhu, X.L. Zhang, Y.L. Song, P. Wu, J. Coord. Chem. 63, 1130 (2010)
R.H. Wang, L. Han, P.L. Jiang, Y.F. Zhou, D.Q. Yuan, M.C. Hong, Cryst. Growth Des. 5, 129 (2005)
M. Xue, G.S. Zhu, Y.J. Zhang, Q.R. Fang, I.J. Hewitt, S.L. Qiu, Cryst. Growth Des. 8, 427 (2008)
C. Biswas, A.D. Jana, M.G.B. Drew, G. Mostafa, A. Ghosh, Polyhedron 28, 653 (2009)
G.P. Yang, Y.Y. Wang, W.H. Zhang, A.Y. Fu, R.T. Liu, E. Lermontova, Q.Z. Shi, Cryst. Eng. Commun. 12, 1509 (2010)
G.C. Liu, Y.Q. Chen, X.L. Wang, B.K. Chen, H.Y. Lin, J. Solid State Chem. 182, 566 (2009)
F.C. Liu, Y.F. Zeng, J. Jiao, X.H. Bu, J. Ribas, S.R. Batten, Inorg. Chem. 45, 2776 (2006)
Z.F. Tian, J.G. Lin, Y. Su, L.L. Wen, Y.M. Liu, H.Z. Zhu, Q.J. Meng, Cryst. Growth Des. 7, 1863 (2007)
J. Yang, J.F. Ma, Y.Y. Liu, J.C. Ma, H.Q. Jia, N.H. Hu, Eur. J. Inorg. Chem. 2006, 1208 (2006)
H.L. Wang, D.P. Zhang, D.F. Sun, Y.T. Chen, L.F. Zhang, L.J. Tian, J.Z. Jiang, Z.H. Ni, Cryst. Growth Des. 9, 5273 (2009)
J.K. Sun, Q.X. Yao, Z.F. Ju, J. Zhang, Cryst. Eng. Commun. 12, 1709 (2010)
H.L. Wang, D.P. Zhang, D.F. Sun, Y.T. Chen, K. Wang, Z.H. Ni, L.J. Tian, J.Z. Jiang, Cryst. Eng. Commun. 12, 1096 (2010)
A.X.S. Bruker, SAINT Software Reference Manual (Madison, WI, 1998)
G.M. Sheldrick, SHELXTL NT Version 5.1. Program for Solution and Refinement of Crystal Structures (University of Göttingen, Germany, 1997)
G.J. Xu, Y.H. Zhao, K.Z. Shao, Y.Q. Lan, X.L. Wang, Z.M. Zu, L.K. Yan, Cryst. Eng. Commun. 11, 1842 (2009)
T.L. Hu, R.Q. Zou, J.R. Li, X.H. Bu, Dalton Trans. 10, 1302 (2008)
L.L. Wen, D.B. Dang, C.Y. Duan, Y.Z. Li, Z.F. Tian, Q.J. Meng, Inorg. Chem. 44, 7161 (2005)
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (20971018), the National Natural Science Foundation of Shandong Province (ZR2010BL010) and Science & Technology Program of Shandong Province (2010GWZ20251).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, X., Jin, M., Qiu, Y. et al. Syntheses and Characterizations of Two One-Dimensional Coordination Polymers Assembled by Dicarboxylate and N-Donor Coligands. J Inorg Organomet Polym 21, 498–503 (2011). https://doi.org/10.1007/s10904-011-9474-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-011-9474-x