Abstract
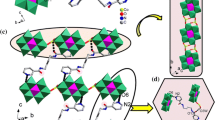
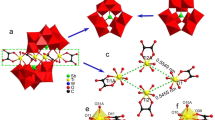
Hydrothermal reactions of 1-aminoethylidenediphosphonic acid (AEDPH4), imidazole (Im) and divalent metal oxides afforded two novel phosphonate compounds: Zn2(AEDP)(Im)3·H2O (1), Cd3(AEDPH)2(Im)5·Im (2). Compounds 1 and 2 are both one-dimensional (1-D) coordination polymers that illustrate different chain motifs. Compound 1 features a zigzag double chain structure while compound 2 has a distorted square-chain structure. Both compounds are further extended to form three-dimensional (3-D) supramolecular structures via hydrogen bonds. The effect of degree of deprotonization of AEDPH4 on the structural types of the final products is also discussed.







Similar content being viewed by others
References
(a) E. Stein, A. Clearfield, and M.A. Subramanian, Solid State Ionics, 83, 113 (1996); (b) G. Alberti and U. Costantino, in Comprehensive Supramolecular Chemistry, ed. By J.-M. Lehn, (Pergamon-Elsevier Science Ltd., London, 1996, p. 1); (c) A Clearfield, Curr. Opin. Solid State Mater. Sci. 1, 268 (1996); (d) A. Clearfield, in Metal phosphonate chemistry in Progress in Inorganic Chemistry, vol 47, ed. by K.D. Karlin (John Wiley & Sons, New York, 1998), pp. 371–510, and references therein
For example (a) J.G. Mao, Z.K. Wang, and A. Clearfield, J. Chem. Soc., Dalton Trans. 23, 4457 (2002); (b) J.L. Song, J.G. Mao, Y.Q. Sun, Z.C. Dong, Eur. J. Inorg. Chem. 23, 4218 (2003); (c) Y. Gong, W. Tang, W.B. Hou, Z.Y. Zha, and C.W. Hu, Inorg. Chem. 45, 4987 (2006); (d) H.H. Song, L.M. Zheng, C.H. Lin, S.L. Wang, X.Q. Xin, S. Gao, Chem. Mater. 11, 2382 (1999); (e) H.H. Song, L.M. Zheng, Z.M. Wang, C.H. Yan, X.Q. Xin, Inorg. Chem. 40, 5024 (2001)
For example; (a) J.G. Mao, Z.K. Wang, A. Clearfield, J. Chem. Soc., Dalton Trans. 23, 4457 (2002); (b) F. Fredoueil, M. Evain, D. Massiot, M. Bujoli, P. Janvier, A. Clearfield, B. Bujoli, J. Chem. Soc., Dalton Trans. 23, 1508 (2002); (c) Z.M. Sun, J.G. Mao, Y.Q. Sun, H.Y. Zeng, A. Clearfield, New J. Chem. 27, 1326 (2003); (d) C.V. Krishnamohan Sharma, C.C. Chusuei, R. Clérac, T. Mǒller, K.R. Dunbar, A. Clearfield, Inorg. Chem. 42, 8300 (2003); (e) D. Grohol, F. Gingl, A. Clearfield, Inorg. Chem. 38, 751 (1999)
For example; (a) J.G. Mao, Z.K. Wang, A. Clearfield, New J. Chem. 26, 1010 (2002); (b) D. Kong, Y. Li, J.H. Ross Jr, A. Clearfield, Chem. Commun. 14, 1720 (2003); (c) J.G. Mao, Z.K. Wang, A. Clearfield, Inorg. Chem. 41, 3713 (2002); (d) C.V. Krishnamohan Sharma, C.C. Chusuei, R. Clérac, T. Mǒller, K.R. Dunbar, A. Clearfield, Inorg. Chem. 42, 8300 (2003); (e) D.K. Cao, Y.Z. Li, Y. Song, L.M. Zheng, Inorg. Chem. 44, 3599 (2004)
(a) P. Yin, X.C. Wang, S. Gao, L.M. Zheng, J. Solid State Chem. 178, 1049 (2000); (b) D.G. Ding, M.C. Yin, H.J. Lu, Y.T. Fan, H.W. Hou, Y.T. Wang. J. Solid State Chem. 179, 747 (2006); (c) M. Li, S.P. Chen, J.F. Xiang, H.J. He, L.J. Yuan, J.T. Sun, Cryst. Growth Des. 6, 1250 (2006); (d) M. Li, J.F. Xiang, S.M. Wu, S.P. Chen, L.J. Yuan, H. Li, H.J. He, J.T. Sun, J. Molecu. Struc. (In press); (e) M. Li, J.F. Xiang, S.P. Chen, S.M. Wu, L.J. Yuan, H. Li, H.J. He, J.T. Sun, J. Coord. Chem. (In press)
B.J. Chai, W. Covina, F.D. Muggee, Anaheim, U.S. Patent 4239695
G.M. Sheldrick, SHEXTL(Xprep, SADABS, XS, XL) Crystallographic Software Package, Version 5.1; Bruker-AXS: Madison, WI, 1998
T.K. Prasad, M.V. Rajasekharan, Inorg. Chem. Commun. 8, 1116 (2005)
D. Kong, A. Clearfield, J. Zoń, Cryst. Growth Des. 5, 1767 (2005)
A. Cabeza, M.A.G. Aranda, S. Bruque, D.M. Poojary, A. Clearfiled, J. Sanz, Inorg. Chem. 37, 4148 (1998)
Acknowledgements
This work was supported by grants of the National Nature Science Foundation of China (No. 20671074).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10904_2007_9154_MOESM1_ESM.doc
MOESM 1: Schematic view of the unit cell linking of compound 1 by hydrogen bonds. Dashed lines represent hydrogen bonds. (DOC 185 kb)
10904_2007_9154_MOESM2_ESM.doc
MOESM 2: Schematic view of the unit cell linking of compound 2 by hydrogen bonds. Dashed lines represent hydrogen bonds. (DOC 221 kb)
Rights and permissions
About this article
Cite this article
Chen, Sp., Li, M., Chen, Ql. et al. Two Novel Metal Phosphonate Compounds: Different One-Dimension Chain Structures Constructed by Imidazole and 1-Aminoethylidenediphosphonic Acid. J Inorg Organomet Polym 17, 665–672 (2007). https://doi.org/10.1007/s10904-007-9154-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-007-9154-z