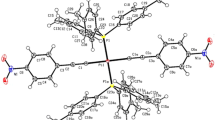
A thermally stable platinum(II) polyyne polymer incorporating carbazole-based linking units, trans-[–Pt(PBu3)2C≡CC≡CRC≡CC≡C–] n (R = 9-butylcarbazole-3,6-diyl), was prepared by polycondensation polymerization of trans-[PtCl2(PBu3)2] with H–C≡CC≡CRC≡CC≡C–H. We report the optical absorption and photoluminescence spectra of this carbon-rich metallopolymer and compare its photophysics with its molecular dinuclear model complex trans-[Pt(Ph)(PEt3)2C≡CC≡CRC≡CC≡CPt(Ph)(PEt3)2] as well as the group 11 gold(I) congener, [(PPh3)AuC≡CC≡CRC≡CC≡CAu(PPh3)]. Characterization of the polymer and metal complexes was accomplished by FT-IR and NMR spectroscopies and FAB mass spectrometry. Our investigations showed that harvesting of the organic triplet emissions can be achieved by the heavy-atom effect of Pt and Au centers, which enables a very high efficiency of intersystem crossing from the S1 singlet excited state to the T1 triplet excited state. The influence of the metal and the C≡C chain length on the intersystem crossing rate and the spatial extent of singlet and triplet excitons is characterized. The present work indicates that high-energy triplet states intrinsically give more efficient phosphorescence in metal-containing diethynylcarbazole systems than in the corresponding bis(butadiynyl) congeners and can thus enhance the radiative decay pathway.










Similar content being viewed by others
REFERENCES
(a) I. F. Perepichka, D. D. Mysyk, and N. I. Sokolov, in Current Trends in Polymer Photochemistry, N. S. Allen, M. Edge, I. R. Bellobono, and E. Selli, eds. (Ellis Horwood, New York, London, 1995), p. 318. (b) J. B. Birks, Photophysics of Aromatic Molecules (Wiley-Interscience, London, 1970), Chap. 7).
(a) P. Strohriegl and J. V. Grazulevicius, Adv. Mater. 14, 1439 (2002). (b) P. Kunda, K. R. Justin Thomas, J. T. Lin, Y.-T. Tao, and C.-H. Chien, Adv. Funct. Mater. 13, 445 (2003). (c) K. R. Justin Thomas, J. T. Lin, Y.-T. Tao, and C.-W. Ko, Adv. Mater. 12, 1949 (2000). (d) K. R. Justin Thomas, J. T. Lin, Y.-T. Tao, and C.-W. Ko, J. Am. Chem. Soc. 123, 9404 (2001). (e) F. Sanda, T. Nakai, N. Kobayashi, and T. Masuda, Macromolecules 37, 2703 (2004). (f) S. Maruyama, X.-T. Tao, H. Hokari, T. Noh, Y. Zhang, T. Wada, H. Sasabe, H. Suzuki, T. Watanabe, and S. Miyata, J. Mater. Chem. 9, 893 (1999). (g) J. Chung, B. Choi, and H. H. Lee, Appl. Phys. Lett. 74, 3645 (1999). (h) X. T. Tao, H. Suzuki, T. Wada, H. Saade, and S. Miyata, Appl. Phys. Lett. 75, 1655 (1999).
(a) B. Kippelen, A. Golemme, E. Hendrickx, J. F. Wang, S. R. Marder, and N. Peyghambarian, in Field Responsive Polymers: Electroresponsive, Photoresponsive, and Responsive Polymers in Chemistry and Biology, I. M. Khan and J. S. Harrison eds. (ACS Symposium Series 726, American Chemical Society, Washington D.C., USA, 1999), p. 204. (b) Y. Z. Wang and A. J. Epstein, Acc. Chem. Res. 32, 217 (1999).
(a) Y. Kuwabara, H. Ogawa, H. Inada, N. Noma, and Y. Shirota, Adv. Mater. 6, 677 (1994). (b) B. E. Koene, D. E. Loy, and M. E. Thompson, Chem. Mater. 10, 2235 (1998). (c) D. F. O’Brien, P. E. Burrows, S. R. Forrest, B. E. Koene, D. E. Loy, and M. E. Thompson, Adv. Mater. 10, 1108 (1998).
Joule J. A., (1984) Adv. Heterocycl. Chem. 35: 83
(a) F. L. Bai, M. Zheng, G. Yu, D. B. Zhu, Thin Solid Films 363, 118 (2000). (b) D. B. Romero, M. Schaer, M. Leclerc, D. Ades, A. Siove, and L. Zuppiroli, Synth. Met. 80, 271 (1996).
(a) B. J. Coe and N. R. M. Curati, Comment Inorg. Chem. 25, 147 (2004). (b) M. A. Baldo, M. E. Thompson, and S. R. Forrest, Pure Appl. Chem. 71, 2095 (1999).
(a) R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. dos Santos, J.-L. Bredas, M. Logdlund, and W. R. Salaneck, Nature 397, 121 (1999). (b) M. A. Baldo, D. F. O’Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson, and S. R. Forrest, Nature 395, 151 (1998). (c) Y. Cao, I. D. Parker, G. Yu, C. Zhang, and A. J. Heeger, Nature 397, 414 (1999).
(a) A. Kimoto, J.-S. Cho, M. Higuchi, and K. Yamamoto, Macromolecules 37, 5531 (2004). (b) Z. Zhu and J. S. Moore, J. Org. Chem. 65, 116 (2000). (c) F. Sanda, T. Kawaguchi, and T. Masuda, Macromolecules 36, 2224 (2003). (d) C. Xia and R. C. Advincula, Macromolecules 34, 5854 (2001). (e) S. Maruyama, H. Hokari, T. Wada, and H. Sasabe, Synthesis 1794 (2001). (f) B. Z. Tang, H. Z. Chen, R. S. Xu, J. W. Y. Lam, K. K. L. Cheuk, H. N. C. Wong, and M. Wang, Chem. Mater. 12, 213 (2000). (g) Z. L. Xie, J. W. Y. Lam, C. F. Qiu, M. Wong, H. S. Kwok, and B. Z. Tang, Polym. Mater. Sci. Eng. 89, 416 (2003).
(a) W.-Y. Wong, Coord. Chem. Rev. 249, 971 (2005). (b) N. D. McClenaghan, R. Passalacqua, F. Loiseau, S. Campagna, B. Verheyde, A. Hameurlaine, and W. Dehaen, J. Am. Chem. Soc. 125, 5356 (2003). (c) A.-C. Ribou, T. Wada, and H. Saabe, Inorg. Chim. Acta 288, 134 (1999).
(a) C.-H. Tao, K. M.-C. Wong, N. Zhu, and V. W.-W. Yam, New J. Chem. 27, 150 (2003). (b) W.-Y. Wong, G.-L. Lu, K.-H. Choi, and J.-X. Shi, Macromolecules 35, 3506 (2002). (c) A. S. Abd-El-Aziz, C. E. Carraher, Jr., C. U. Pittman, Jr., J. E. Sheats, and M. Zeldin, Macromolecules Containing Metal and Metal-Like Elements (Wiley-Interscience, New Jersey, 2003), Vol. 1, Chap. 4, p. 158.
(a) W.-Y. Wong, C.-K. Wong, G.-L. Lu, A. W.-M. Lee, K.-W. Cheah, and J.-X. Shi, Macromolecules 36, 983 (2003). (b) W.-Y. Wong, L. Liu, S.-Y. Poon, A. W.-M. Lee, K.-W. Cheah, and J.-X. Shi, Macromolecules 37, 4496 (2004). (c) W.-Y. Wong, S.-Y. Poon, A. W.-M. Lee, J.-X. Shi, and K.-W. Cheah, Chem. Commun. 2420 (2004).
(a) W.-Y. Wong, J. Inorg. Organomet. Polym. Mater. 15, 197 (2005). (b) N. J. Long, Angew. Chem. Int. Ed. Engl. 34, 21 (1995). (c) A. S. Abd-El-Aziz, Macromol. Rapid Commun. 23, 995 (2002).
J. Chatt and B. L. Shaw, J. Chem. Soc. 4020 (1960).
J. Chatt and R. G. Hayter, J. Chem. Soc. Dalton Trans. 896 (1961).
(a) W. R. Dawson and M. W. Windsor, J. Phys. Chem. 72, 3251 (1968). (b) J. S. Wilson, N. Chawdhury, M. R. A. Al-Mandhary, M. Younus, M. S. Khan, P. R. Raithby, A. Köhler, and R. H. Friend, J. Am. Chem. Soc. 123, 9412 (2001).
SAINT+, ver. 6.02a, Bruker Analytical X-ray System, Inc. (Madison, Wisconsin, USA, 1998).
G. M. Sheldrick, SADABS, Empirical Absorption Correction Program, University of Göttingen, Germany (1997).
G. M. Sheldrick, SHELXTLTM, Reference Manual, ver. 5.1 (Madison, Wisconsin, USA 1997).
(a) N. Chawdhury, A. Köhler, R. H. Friend, W.-Y. Wong, J. Lewis, M. Younus, P. R. Raithby, T. C. Corcoran, M. R. A. Al-Mandhary, and M. S. Khan, J. Chem. Phys. 110, 4963 (1999). (b) M. Younus, A. Köhler, S. Cron, N. Chawdhury, M. R. A. Al-Mandhary, M. S. Khan, J. Lewis, N. J. Long, R. H. Friend, and P. R. Raithby, Angew. Chem. Int. Ed. 37, 3036 (1998). (c) W.-Y. Wong, S.-M. Chan, K.-H. Choi, K.-W. Cheah, and W.-K. Chan, Macromol. Rapid Commun. 21, 453 (2000). (d) W.-Y. Wong, K.-H. Choi, G.-L. Lu, Macromol. Rapid Commun. 22, 461 (2001).
(a) W.-Y. Wong, K.-H. Choi, G.-L. Lu, J.-X. Shi, P.-Y. Lai, S.-M. Chan, and Z. Lin, Organometallics 20, 5446 (2001). (b) B. Li, B. Ahrens, K.-H. Choi, M. S. Khan, P. R. Raithby, P. J. Wilson, and W.-Y. Wong, Cryst. Eng. Commun. 4, 405 (2002). (c) W.-Y. Wong, Comment Inorg. Chem. 26, 39 (2005). (d) R. J. Puddephatt, Coord. Chem. Rev. 216–217, 313 (2001). (e) R. J. Puddephatt, Chem. Commun. 1055 (1998).
(a) U. H. F. Bunz, Chem. Rev. 100, 1605 (2000). (b) I. Manners, Synthetic Metal-Containing Polymers (Wiley-VCH, Weinheim, Germany, 2004), Chap. 5.
Chawdhury N., Köhler A., Friend R. H., Younus M., Long N. J., Raithby P. R., Lewis J., (1998) Macromolecules 31: 722
(a) S. D. Cummings and R. Eisenberg, J. Am. Chem. Soc. 118, 1949 (1996). (b) N. J. Demas and G. A. Crosby, J. Am. Chem. Soc. 92, 7262 (1970).
N. J. Turro, Modern Molecular Photochemistry (University Science Books, Mill Valley, California, USA, 1991).
ACKNOWLEDGMENTS
Financial support from the Hong Kong Research Grants Council (HKBU 2022/03P) and Hong Kong Baptist University (FRG/03-04/II-69) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Richard J. Puddephatt in recognition of his outstanding contribution to inorganic and organometallic chemistry.
Rights and permissions
About this article
Cite this article
Liu, L., Wong, WY., Poon, SY. et al. Synthesis, Characterization and Optoelectronic Properties of Dimeric and Polymeric Metallaynes Derived from 3,6-Bis(buta-1,3-diynyl)-9-butylcarbazole. J Inorg Organomet Polym 15, 555–567 (2005). https://doi.org/10.1007/s10904-006-9009-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-006-9009-z