Abstract
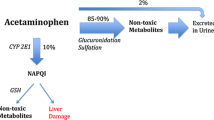
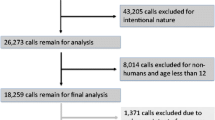
The U.S. Food and Drug Administration has formally requested that pharmaceutical manufacturers limit the amount of acetaminophen (APAP) in prescription products. The goal of this study was to describe a state-wide poison control system’s experience with prescription APAP ingestions that resulted in hepatic injury or death. Retrospective analysis of statewide California Poison Control System electronic database for cases from the years 2000 through 2009. Inclusion criteria: adults ≥18 years of age following therapeutic use of an acetaminophen-containing prescription with laboratory evidence of liver injury. Forty cases met the inclusion criteria. All cases involved at least two concurrent acetaminophen-containing products. Prescription APAP strengths of 500 mg per tablet or greater were involved in 32 of 40 cases (80 %). Thirty patients survived, three died, one underwent liver transplantation and seven cases were lost to follow up. The ingestion of concentrated prescription APAP-containing medications in combination with other sources of APAP can result in severe liver injury and death. Limiting the amount of APAP from prescription medications in conjunction with enhanced prescriber and patient education regarding the hazards of ingesting multiple APAP-containing products may assist in decreasing the overall incidence of unintentional APAP poisonings in the United States. Future prospective studies are required to determine if these measures will have a significant impact on both the morbidity and mortality associated with APAP administration.
Similar content being viewed by others
References
Black, M. (1984). Acetaminophen hepatoxicity. Annual Review of Medicine, 35, 577–593.
Schiodt, F. V., Rochling, F. A., Casey, D. L., et al. (1997). Acetaminophen toxicity in an urban county hospital. New England Journal of Medicine, 337, 1112–1117.
Larson, A. M., Polson, J., Fontana, R. J., et al. (2005). Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology, 42(6), 1364–1372.
Craig, D. G., Bates, C. M., Davidson, J. S., Martin, K. G., Hayes, P. C., & Simpson, K. J. (2011). Overdose pattern and outcome in paracetamol-induced acute severe hepatotoxicity. British Journal of Clinical Pharmacology, 71(2), 273–282.
United States Food and Drug Administration. FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit. http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm (accessed 2011 Oct 20).
Green, T. J., Sivilotti, M. L., Langmann, C., et al. (2010). When do the aminotransferases rise after acute acetaminophen overdose? Clinical toxicology, 48(8), 787–792.
Bronstein, A. C., Spyker, D. A., Cantilena, L. R., et al. (2009). 2008 Annual Report of the American Association of Poison Control Center’s National Poison Data System: 26th Annual Report. Clinical toxicology, 47(10), 911–1084.
Drug Topics. Drug Topics releases top branded and generic drug lists. http://drugtopics.modernmedicine.com/drugtopics/article/articleDetail.jsp?id=727261 (accessed 2011 Oct 20).
Dart, R. C., Erdman, A. R., Olson, K. R., et al. (2006). Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clinical toxicology (Philadelphia, Pa.), 44(1), 1–18.
Harrison, P. M., Keays, R., Bray, G. P., et al. (1990). Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet, 335, 1572–1573.
Keays, R., Harrison, P. M., Wendon, J. A., et al. (1991). Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trail. BMJ, 303(6809), 1026–1029.
Hornsby, L. B., Whitley, H. P., Hester, E. K., et al. (2010). Survey of patient knowledge related to acetaminophen recognition, dosing, and toxicity. Journal American Pharmacists Association, 50(4), 485–492.
Fosnocht, D., Taylor, J. R., & Caravati, E. M. (2008). Emergency department knowledge concerning acetaminophen (paracetamol) in over-the-counter and prescription analgesics. Emergency Medical Journal, 25(4), 213–216.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez, R.M., Nordt, S.P. & Cantrell, F.L. Prescription Acetaminophen Ingestions Associated with Hepatic Injury and Death. J Community Health 37, 1249–1252 (2012). https://doi.org/10.1007/s10900-012-9563-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10900-012-9563-y