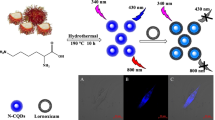
Abstract
In this study, a novel and simple fluorescent carbon quantum dots (CQDs) based nano-sensor for colchicine determination has been prepared. The nitrogen doped CQDs probe was prepared using uric acid as a carbon/nitrogen source via a one-step pyrolysis. The sensor is based on inner filter effect (IFE) where colchicine acts as a powerful absorber that affects the excitation of the fluorescer (CQDs). This overlap results in a quantitative attenuation of the fluorescence of CQDs with increasing colchicine concentration in the range of 2–25 μM. The developed sensor has the advantages of simplicity, less time-consuming, convenience and satisfactory selectivity for colchicine determination in pharmaceutical dosage forms.









Similar content being viewed by others
References
Leung YY, Yao Hui LL, Kraus VB (2015) Colchicine--update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 45(3):341–350. https://doi.org/10.1016/j.semarthrit.2015.06.013
Cabău G, Crișan TO, Klück V, Popp RA, Joosten LAB (2020) Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunol Rev 294(1):92–105. https://doi.org/10.1111/imr.12833
Dasgeb B, Kornreich D, McGuinn K, Okon L, Brownell I, Sackett DL (2018) Colchicine: an ancient drug with novel applications. Br J Dermatol 178(2):350–356. https://doi.org/10.1111/bjd.15896
Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F (2019) Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 381(26):2497–2505. https://doi.org/10.1056/NEJMoa1912388
Gracheva IA, Shchegravina ES, Schmalz H-G, Beletskaya IP, Fedorov AY (2020) Colchicine alkaloids and synthetic analogues: current progress and perspectives. J Med Chem 63(19):10618–10651. https://doi.org/10.1021/acs.jmedchem.0c00222
Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, Pollak U, Koren G, Bentur Y (2010) Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol 48(5):407–414. https://doi.org/10.3109/15563650.2010.495348
Mohamed A-MI, Omar MA, Derayea SM, Hammad MA, Mohamed AA (2018) Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations. Open Chem 16(1):496–510. https://doi.org/10.1515/chem-2018-0050
Bodoki E, Oprean R, Vlase L, Tamas M, Sandulescu R (2005) Fast determination of colchicine by TLC-densitometry from pharmaceuticals and vegetal extracts. J Pharm Biomed Anal 37(5):971–977. https://doi.org/10.1016/j.jpba.2004.10.006
Fahim M, Singh M, Kamal YT, Mukhtar HM, Ahmad S (2015) A high performance thin layer chromatographic method for the estimation of colchicine in different formulations. J Pharm Bioallied Sci 7(4):260–263. https://doi.org/10.4103/0975-7406.168021
Hadad GM, Badr JM, El-Nahriry K, Hassanean HA (2012) Validated HPLC and HPTLC methods for simultaneous determination of colchicine and khellin in pharmaceutical formulations. J Chromatogr Sci 51(3):258–265. https://doi.org/10.1093/chromsci/bms135
Mohammed S, Abd El SA, Mohammed IA (2019) HPLC, densitometric and spectrophotometric methods for the simultaneous determination of colchicine and probenecid in their binary mixture. Asian J Appl Chem Res 2(2):1–12. https://doi.org/10.9734/ajacr/2018/v2i229673
Çankaya N, Bulduk İ, Çolak AM (2019) Extraction, development and validation of HPLC-UV method for rapid and sensitive determination of colchicine from Colchicum autumnale L. Bulbs. Saudi J Biol Sci 26(2):345–351. https://doi.org/10.1016/j.sjbs.2018.10.003
Ibrahim MA, Yusrida D, AKK N, Reem Abou A, Gabriel Onn Kit L (2017) A simple (HPLC-UV) method for the quantification of colchicine in bulk and ethosomal gel nano-formulation and its validation. Int J Pharm Pharm Sci 9(7):72–78. https://doi.org/10.22159/ijpps.2017v9i7.18336
Rica CI, Naessens T, Pieters L, Apers S (2017) An HPLC method for the quantification of colchicine and colchicine derivatives in gloriosa superba seeds. Nat prod Commun 12(8):1215–1221. https://doi.org/10.1177/1934578x1701200817
Abdel Razeq SA, Khalil IA, Mohammed SA (2020) Stability-indicating chromatographic and UV spectrophotometric methods for determination of colchicine. J Iran Chem Soc 17(9):2415–2427. https://doi.org/10.1007/s13738-020-01937-8
Fabresse N, Allard J, Sardaby M, Thompson A, Clutton RE, Eddleston M, Alvarez J-C (2017) LC–MS/MS quantification of free and fab-bound colchicine in plasma, urine and organs following colchicine administration and colchicine-specific fab fragments treatment in Göttingen minipigs. J Chromatogr B 1060:400–406. https://doi.org/10.1016/j.jchromb.2017.06.034
Jiang Y, Wang J, Wang Y, Li H, Fawcett JP, Gu J (2007) Rapid and sensitive liquid chromatography-tandem mass spectrometry method for the quantitation of colchicine in human plasma. J Chromatogr B 850(1–2):564–568. https://doi.org/10.1016/j.jchromb.2006.12.022
Gul H, Demirkaya E, Eser B, Kapucu H, Tuncbilek V, Simsek D (2015) Colchicine measurement using LC-MS/MS with ESI in serum with liquid liquid extraction. Pediatr Rheumatol 13(S1):118. https://doi.org/10.1186/1546-0096-13-S1-P118
Wang F, Zhou J, Liu Y, Wu S, Song G, Ye B (2011) Electrochemical oxidation behavior of colchicine on a graphene oxide-Nafion composite film modified glassy carbon electrode. Analyst 136(19):3943–3949. https://doi.org/10.1039/C1AN15372B
Zhang H (2006) Electrochemistry and voltammetric determination of colchicine using an acetylene black-dihexadecyl hydrogen phosphate composite film modified glassy carbon electrode. Bioelectrochemistry 68(2):197–201. https://doi.org/10.1016/j.bioelechem.2005.07.001
Afzali M, Mostafavi A, Shamspur T (2020) Sensitive detection of colchicine at a glassy carbon electrode modified with magnetic ionic liquid/CuO nanoparticles/carbon nanofibers in pharmaceutical and plasma samples. J Iran Chem Soc 17(7):1753–1764. https://doi.org/10.1007/s13738-020-01894-2
Suad KO, Abdolla NSY, Boujwod FF, Yaman MMR (2019) Spectrophotometric method for the determination of colchicine in pure and pharmaceutical forms (a kinetic study). J Pure Appl Sci 18(4):313–319
Liu YM, Guizhi L (2000) Determination of colchicine in tablet by fluorimetry. Chin J Anal Chem 38(3):331–332
Ngororabanga JMV, Du Plessis J, Mama N (2017) Fluorescent polymer incorporating triazolyl coumarin units for cu(2+) detection via planarization of Ict-based fluorophore. Sensors 17(9):1–13. https://doi.org/10.3390/s17091980
Liu Y, Huang H, Cao W, Mao B, Liu Y, Kang Z (2020) Advances in carbon dots: from the perspective of traditional quantum dots. Mater Chem Front 4(6):1586–1613. https://doi.org/10.1039/D0QM00090F
Molaei MJ (2020) Principles, mechanisms, and application of carbon quantum dots in sensors: a review. Anal Methods 12(10):1266–1287. https://doi.org/10.1039/C9AY02696G
Magdy G, Abdel Hakiem AF, Belal F, Abdel-Megied AM (2021) Green one-pot synthesis of nitrogen and sulfur co-doped carbon quantum dots as new fluorescent nanosensors for determination of salinomycin and maduramicin in food samples. Food Chem 343:128539. https://doi.org/10.1016/j.foodchem.2020.128539
Vázquez-González M, Carrillo-Carrion C (2014) Analytical strategies based on quantum dots for heavy metal ions detection. J Biomed Opt 19(10):101503. https://doi.org/10.1117/1.JBO.19.10.101503
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184(7):1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Pan M, Xie X, Liu K, Yang J, Hong L, Wang S (2020) Fluorescent carbon quantum dots-synthesis, functionalization and sensing application in food analysis. Nanomaterials 10(5):930–954. https://doi.org/10.3390/nano10050930
Qin J, Zhang L, Yang R (2019) Powder carbonization to synthesize novel carbon dots derived from uric acid for the detection of Ag(I) and glutathione. Spectrochim Acta A 207:54–60. https://doi.org/10.1016/j.saa.2018.08.066
Rurack K (2008) Fluorescence quantum yields: methods of determination and standards. In: Resch-Genger U (ed) Standardization and quality Assurance in Fluorescence Measurements, vol 5. Springer, Berlin, pp 101–145
Li P, Hu Y (2018) “Turn-off” fluorescent sensor for pamidronate disodium and zoledronic acid based on newly synthesized carbon dots from black tea. J Anal Methods Chem 2018:3631249–3631247. https://doi.org/10.1155/2018/3631249
Li H, Kong W, Liu J, Liu N, Huang H, Liu Y, Kang Z (2015) Fluorescent N-doped carbon dots for both cellular imaging and highly-sensitive catechol detection. Carbon 91:66–75. https://doi.org/10.1016/j.carbon.2015.04.032
Chen S, Yu Y-L, Wang J-H (2018) Inner filter effect-based fluorescent sensing systems: a review. Anal Chim Acta 999:13–26. https://doi.org/10.1016/j.aca.2017.10.026
ICH harmonised tripartite guidelines (2005) validation of analytical procedures: Text and methodology Q2(R1). http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 15 Nov 2020
British Pharmacopoeia Commission (2016) British pharmacopoeia 2016: volume III. The Stationary Office (TSO), London
Availability of Data and Materials
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Samah F. El-Malla], [Eman A. Elshenawy], [Sherin F. Hammad], [Fotouh R. Mansour]; Formal analysis [Samah F. El-Malla], [Eman A. Elshenawy], [Sherin F. Hammad], [Fotouh R. Mansour]; Investigation: [Eman A. Elshenawy]; Writing - original draft: [Eman A. Elshenawy]; Writing - review and editing: [Samah F. El-Malla], [Sherin F. Hammad], [Fotouh R. Mansour]. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Malla, S.F., Elshenawy, E.A., Hammad, S.F. et al. N-Doped Carbon Dots as a Fluorescent Nanosensor for Determination of Colchicine Based on Inner Filter Effect. J Fluoresc 31, 675–684 (2021). https://doi.org/10.1007/s10895-021-02698-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02698-0