Abstract
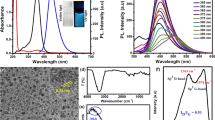
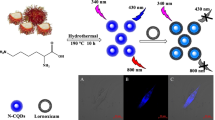
Nitrogen doped carbon quantum dots (NCQDs) were synthesized via hydrothermal route. The NCQDs are thermally and optically stable with high flouresence yield. For the synthesis of NCQDs, citric acid and urea was taken as carbon and nitrogen sources, respectively. The Transmission Electron Microscopy (TEM) of these quantum dots revealed nearly spherical shape and average size of 1.5 nm, which was calculated using Image J software. The quantum dots were also well-characterized using spectroscopic techniques such as FTIR, UV-Visible absorption and fluorescence. These synthesized and characterized dots were utilized for selective detection of lactose in Milli Q water. The bioprobe provide a wide linear range varying from (10.00–77.41) μM with limit of detection 11.36 μM and sensitivity equal to (0.0065 ± 0.0002) μM−1.

Graphical Abstract





Similar content being viewed by others
References
Heyman MB (2006) Lactose intolerance in infants, children, and adolescents. Pediatrics 118(3):1279–1286
Vesa TH, Marteau P, Korpela R (2000) Lactose intolerance. J Am Coll Nutr 19(sup2):165S–175S
Dwevedi A, Singh AK, Singh DP, Srivastava ON, Kayastha AM (2009) Lactose nano-probe optimized using response surface methodology. Biosens Bioelectron 25(4):784–790
Deng Y, Misselwitz B, Dai N, Fox M (2015) Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 7(9):8020–8035
Saha M, Parveen I, Shil BC, Saha SK, Banik RK, Majumder M, Salam MU, Islam AN (2016) Lactose intolerance and symptom pattern of lactose intolerance among healthy volunteers. Euroasian J Hepatogastroenterol 6(1):5–7
Kishore D, Talat M, Srivastava ON, Kayastha AM (2012) Immobilization of β-galactosidase onto functionalized graphene nano-sheets using response surface methodology and its analytical applications. PLoS One 7(7):e40708
Lynch JM, Barbano DM, Fleming JR (2007) Determination of the lactose content of fluid milk by spectrophotometric enzymatic analysis using weight additions and path length adjustment: collaborative study. J AOAC Int 90(1):196–216
Colinas C, Barrera I, Blanco CA (2006) A novel correlation for rapid lactose determination in milk by a cryoscopic technique. J AOAC Int 89(6):1581–1584
Geisser A, Hendrich T, Boehm G, Stahl B (2005) Separation of lactose from human milk oligosaccharides with simulated moving bed chromatography. J Chromatogr A 1092(1):17–23
Kucerova P, Komenska P, Tomkova H, Skopalova J, Bartak P (2017) Determination of lactose in milk products: a comparison of three-enzyme amperometric biosensor and gas chromatography/tandem mass spectrometry. Monatsh Chem 148(3):517–524
Marrakchi M, Dzyadevych SV, Lagarde F, Martelet C, Jaffrezic-Renault N (2008) Conductometric biosensor based on glucose oxidase and beta-galactosidase for specific lactose determination in milk. Mat Sci Eng C-Bio S 28(5–6):872–875
Soldatkin OO, Peshkova VM, Saiapina OY, Kucherenko IS, Dudchenko OY, Melnyk VG, Vasylenko OD, Semenycheva LM, Soldatkin AP, Dzyadevych SV (2013) Development of conductometric biosensor array for simultaneous determination of maltose, lactose, sucrose and glucose. Talanta 115:200–207
Pfeiffer D, Ralis EV, Makower A, Scheller FW (1990) Amperometric Bienzyme based biosensor for the detection of lactose - characterization and application. J Chem Technol Biotechnol 49(3):255–265
Singh AK, Prakash P, Singh R, Nandy N, Firdaus Z, Bansal M, Singh RK, Srivastava A, Roy JK, Mishra B, Singh RK (2017) Curcumin quantum dots mediated degradation of bacterial biofilms. Front Microbiol 8:1517
Singh R, Kashayap S, Singh V, Kayastha AM, Mishra H, Saxena PS, Srivastava A, Singh RK (2018) QPRTase modified N-doped carbon quantum dots: a fluorescent bioprobe for selective detection of neurotoxin quinolinic acid in human serum. Biosens Bioelectron 101:103–109
Miao P, Tang Y, Han K, Wang B (2015) Facile synthesis of carbon nanodots from ethanol and their application in ferric(iii) ion assay. J Mater Chem A 3(29):15068–15073
Dai H, Shi Y, Wang Y, Sun Y, Hu J, Ni P, Li Z (2014) A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sensors Actuators B Chem 202:201–208
Jiang G, Jiang T, Zhou H, Yao J, Kong X (2015) Preparation of N-doped carbon quantum dots for highly sensitive detection of dopamine by an electrochemical method. RSC Adv 5(12):9064–9068
Xu Q, Pu P, Zhao J, Dong C, Gao C, Chen Y, Chen J, Liu Y, Zhou H (2015) Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(iii) detection. J Mater Chem A 3(2):542–546
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed Eng 49(38):6726–6744
Dong Y, Li G, Zhou N, Wang R, Chi Y, Chen G (2012) Graphene quantum dot as a green and facile sensor for free chlorine in drinking water. Anal Chem 84(19):8378–8382
Karfa P, Roy E, Patra S, Kumar S, Tarafdar A, Madhuri R, Sharma PK (2015) Amino acid derived highly luminescent, heteroatom-doped carbon dots for label-free detection of Cd2+/Fe3+, cell imaging and enhanced antibacterial activity. RSC Adv 5(72):58141–58153
Liao J, Cheng Z, Zhou L (2016) Nitrogen-doping enhanced fluorescent carbon dots: green synthesis and their applications for bioimaging and label-free detection of Au3+ ions. ACS Sustain Chem Eng 4(6):3053–3061
Yu J, Xu C, Tian Z, Lin Y, Shi Z (2016) Facilely synthesized N-doped carbon quantum dots with high fluorescent yield for sensing Fe3+. New J Chem 40(3):2083–2088
Li L, Li L, Wang C, Liu K, Zhu R, Qiang H, Lin Y (2015) Synthesis of nitrogen-doped and amino acid-functionalized graphene quantum dots from glycine, and their application to the fluorometric determination of ferric ion. Microchim Acta 182(3):763–770
Li Q, Ohulchanskyy TY, Liu R, Koynov K, Wu D, Best A, Kumar R, Bonoiu A, Prasad PN (2010) Photoluminescent carbon dots as biocompatible Nanoprobes for targeting Cancer cells in vitro. J Phys Chem C 114(28):12062–12068
Zhang R, Chen W (2014) Nitrogen-doped carbon quantum dots: facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens Bioelectron 55:83–90
Yadav A, Pandey SK, Agrawal DC, Mishra H, Srivastava A, Kayastha AM (2019) Carbon nanotubes molybdenum disulfide 3D nanocomposite as novel nanoscaffolds to immobilize Lens culinaris β-galactosidase (Lsbgal): robust stability, reusability, and effective bioconversion of lactose in whey. Food Chem 297:125005
Sarkar P, Nicholson PS (1996) Electrophoretic deposition (EPD): mechanisms, kinetics, and application to ceramics. J Am Ceram Soc 79(8):1987–2002
Li S, Li Y, Cao J, Zhu J, Fan L, Li X (2014) Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal Chem 86(20):10201–10207
Dong Y, Chen C, Lin J, Zhou N, Chi Y, Chen G (2013) Electrochemiluminescence emission from carbon quantum dot-sulfite coreactant system. Carbon 56:12–17
Singh R, Rai SK, Tiwari MK, Mishra A, Tewari AK, Mishra PC, Singh RK (2017) An excellent stable fluorescent probe: selective and sensitive detection of trace amounts of Hg+2 ions in natural source of water. Chem Phys Lett 676:39–45
Tkac J, Sturdik E, Gemeiner P (2000) Novel glucose non-interference biosensor for lactose detection based on galactose oxidase-peroxidase with and without co-immobilised [small beta]-galactosidase. Analyst 125(7):1285–1289
Rajendran V, Irudayaraj J (2002) Detection of glucose, galactose, and lactose in Milk with a microdialysis-coupled flow injection Amperometric sensor. J Dairy Sci 85(6):1357–1361
Ammam M, Fransaer J (2010) Two-enzyme lactose biosensor based on beta-galactosidase and glucose oxidase deposited by AC-electrophoresis: characteristics and performance for lactose determination in milk. Sensor Actuat B-Chem 148(2):583–589
Acknowledgements
Anjali Yadav is grateful to UGC, New Delhi, for fellowship in the form of junior research fellowship and senior research fellowship (F./2015-16/NFO-2015-17-OBC-UTT-32658/ (SA-III-website). Ranjana Singh is grateful to CSIR New Delhi, Govt. of India for providing financial support in terms of CSIR Research Associate ship with file no 09/13 (0846)/2018-EMR-I dated 29/03/2019. Shashank Shekhar want to acknowledge CSIR (09/013(0739)/2017-EMR-I), New Delhi for financial support. Ranjan K. Singh is grateful to AvH Foundation, Germany, DST, India for FIST programme. Authors are extremely thankful to Biophysics Laboratory, Department of Physics, B.H.U. Varanasi, India to access the Edinburg FL 900 fluorescence spectrophotometer for fluorescence measurement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interests
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Sensitive and selective detection of lactose using fluorescent bioprobe.
• Amide bond formation between Lsbgal and nitrogen doped carbon quantum dots (NCQDs)
• Static quenching of the fluorescent bioprobe after addition of different amounts of lactose prepared in the Milli Q water.
Electronic supplementary material
ESM 1
(DOCX 8428 kb)
Rights and permissions
About this article
Cite this article
Singh, R., Yadav, A., Shekhar, S. et al. Nitrogen Doped Carbon Quantum Dots Modified by Lens culinaris β-Galactosidase as a Fluorescent Probe for Detection of Lactose. J Fluoresc 29, 1213–1219 (2019). https://doi.org/10.1007/s10895-019-02430-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02430-z