Abstract
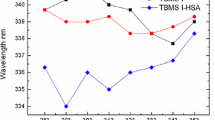
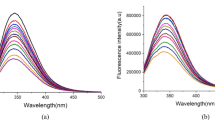
Swertisin (6-glucosyl flavonoid) and spinosin (2″-β-O-glucopyranosyl swertisin) are two main components from Ziziphi Spinosae Semen, with anti-anxiety and hypnosis effects. The paper aims to compare the differences between the two compounds binding with serum albumins (BSA and HSA). Swertisin and spinosin statically quench intrinsic fluorescence of serum proteins by binding to proteins to form complexes. The fluorescence quenching rates of BSA induced by swertisin or spinosin are faster than those of HSA resulted by swertisin or spinosin, respectively. Each serum protein has only one binding site respectively accessible to the two compounds. Hydrophobic force and hydrogen bond play the important roles during the biding process of swertisin with proteins, but van der Waals force and hydrogen bond are major driving forces for spinosin binding to proteins. Synchronous fluorescence data show that spinosin binds to BSA and HSA and thus changes Tyr and Trp residue microenvironments, and has a greater effect on the latter. Compared with swertisin, spinosin has a stronger effect on the α-helix of proteins. But the distance between swertisin and proteins is slightly closer than spinosin. These findings will contribute to further understand the reaction of Ziziphi Spinosae Semen in the liver phase I oxidation, intestinal hydrolysis and deparaffin metabolism.







Similar content being viewed by others
References
Li H, Wu F, Tan J, Wang K, Zhang C, Zheng H, Hu F (2016) Caffeic acid phenethyl ester exhibiting distinctive binding interaction with human serum albumin implies the pharmacokinetic basis of propolis bioactive components. J Pharm Biomed Anal 122:21–28
Liu X, Ling Z, Zhou X, Ahmad F, Zhou Y (2016) Comprehensive spectroscopic probing the interaction and conformation impairment of bovine serum albumin (BSA) by herbicide butachlor. J Photochem Photobiol B 162:332–339
Leboffe L, Di MA, Trezza V, Polticelli F, Ascenzi P (2017) Human serum albumin: a modulator of cannabinoid drugs. IUBMB Life 69(11):834–840
Hekmat A, Hajebrahimi Z, Motamedzade A (2017) Structural changes of human serum albumin (HSA) in simulated microgravity. Protein Pept Lett 24(11):1030–1039
Ishima Y, Maruyama T (2016) Human serum albumin as carrier in drug delivery systems. Yakuqaku Zasshi 136(1):39–47
Shahabadi N, Maghsudi M, Kiani Z, Pourfoulad M (2011) Multispectroscopic studies on the interaction of 2- tert -butylhydroquinone (TBHQ), a food additive, with bovine serum albumin. Food Chem 124(3):1063–1068
Sekula B, Zielinski K, Bujacz A (2013) Crystallographic studies of the complexes of bovine and equine serum albumin with 3,5-diiodosalicylic acid. Int J Biol Macromol 60(6):316–324
Suryawanshi VD, Walekar LS, Gore AH, Anbhule PV, Kolekar GB (2016) Spectroscopic analysis on the binding interaction of biologically active pyrimidine derivative with bovine serum albumin. J Pharm Anal 6(1):56–63
Li En W, Xue Qiong Z, Yan Qi Y, Yong He Z (2012) Augmentative effect of spinosin on pentobarbital-induced loss of righting reflex in mice associated with presynaptic 5-HT1A receptor. J Pharm Pharmacol 64(2):277–282
Yan Y, Li Q, Du CH, Jia JP, Feng HX, Qin XM (2017) Investigation of the potentially effective components of semen Ziziphi Spinosae based on “in vitro to in vivo” translation approach. Yaoxuexuebao 52(02):283–290
Yan Y, Zhang M, Cui XF, Zhang FS, Gao XX, Guo XD, Du CH, Qin XM (2019) Discussion on research ideas for process in vivo of chemical compositions from Ziziphi Spinosae Semen and its quality marker. Chin Tradit Herb Drugs 50(2):299–309
Zhang Y, Zhang T, Wang F, Xie J (2015) Brain tissue distribution of Spinosin in rats determined by a new high-performance liquid chromatography–electrospray ionization–mass/mass spectrometry method. J Chromatogr Sci 53(1):97–103
Liu J, Zhai WM, Yang YX, Shi JL, Liu QT, Liu GL, Fang N, Li J, Guo JY (2015) GABA and 5-HT systems are implicated in the anxiolytic-like effect of spinosin in mice. Pharmacol Biochem Behav 128:41–49
Wang LE, Bai YJ, Shi XR, Cui XY, Cui SY, Zhang F, Zhang QY, Zhao YY, Zhang YH (2008) Spinosin, a -glycoside flavonoid from semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacol Biochem Behav 90(3):399–403
Oh HK, Jeon SJ, Lee S, Lee HE, Kim E, Park SJ, Kim HN, Jung WY, Cheong JH, Jang DS (2016) Swertisin ameliorates pre-pulse inhibition deficits and cognitive impairment induced by MK-801 in mice. J Psychopharmacol 31(2):250–259
Lee HE, Jeon SJ, Ryu B, Park SJ, Ko SY, Lee Y, Kim E, Lee S, Kim H, Jang DS (2016) Swertisin, a C-glucosylflavone, ameliorates scopolamine-induced memory impairment in mice with its adenosine A1 receptor antagonistic property. Behav Brain Res 306:137–145
Srivastava A, Dadheech N, Vakani M, Gupta S (2018) Swertisin ameliorates diabetes by triggering pancreatic progenitors for islet neogenesis in Streptozotocin treated BALB/c mice. Biomed Pharmacother 100:221–225
Li Y, Guo Q, Yan Y, Chen T, Du C, Du H (2019) Different effects of Forsythia suspensa metabolites on bovine serum albumin (BSA). Spectrochim Acta A Mol Biomol Spectrosc 214:309–319
Yan X, Chen T, Zhang L, Du H (2018) Study of the interactions of forsythiaside and rutin with acetylcholinesterase (AChE). Int J Biol Macromol 119:1344–1152
Miller JN, Fell AF (1980) The characterization of proteins by synchronous and derivative luminescence spectroscopy. J Pharm Pharmacol 32:70P
Siddiqui GA, Siddiqi MK, Khan RH, Naeem A (2018) Probing the binding of phenolic aldehyde vanillin with bovine serum albumin: evidence from spectroscopic and docking approach. Spectrochim Acta A Mol Biomol Spectrosc 203:40–47
Ashoka S, Seetharamappa J, Kandagal PB, Shaikh SMT (2006) Investigation of the interaction between trazodone hydrochloride and bovine serum albumin. J Lumin 121(1):179–186
Yousefi R, Jamshidi M, Shahsavani MB, Nabavizadeh SM, Haghighi MG, Rashidi M, Taheri-Kafrani A, Niazi A, Keshavarz F, Alavinamehr MM (2016) Study on the interaction of three structurally related cationic Pt(II) complexes with human serum albumin: importance of binding affinity and denaturing properties. J Iran Chem Soc 13(4):617–630
Tang J, Luan F, Chen X (2006) Binding analysis of glycyrrhetinic acid to human serum albumin: fluorescence spectroscopy, FTIR, and molecular modeling. Bioorg Med Chem 14(9):3210–3217
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20(11):3096–3102
Anand U, Jash C, Mukherjee S (2010) Spectroscopic probing of the microenvironment in a protein-surfactant assembly. J Phys Chem B 114(48):15839–15845
Khade BS, Mathe VL, Dongre PM (2017) α-Amylase binding to thermal plasma synthesized zinc oxide nanosheets: a fluorescence study. J Lumin 187:449–456
Klotz IM (2010) Physiochemical aspects of drug-protein interactions: a general perspective. Ann N Y Acad Sci 226(1):18–35
Desfrançois C, Carles S, Schermann JP (2000) Weakly bound clusters of biological interest. Chem Rev 100(11):3943–3962
Pitera JW, Gunsteren WF (2001) The importance of solute−solvent van der Waals interactions with interior atoms of biopolymers. J Am Chem Soc 123(13):3163–3164
Zhang YZ, Zhou B, Liu YX, Zhou CX, Ding XL, Liu Y (2008) Fluorescence study on the interaction of bovine serum albumin with P-Aminoazobenzene. J Fluoresc 18(1):109–118
Shi JH, Chen J, Wang J, Zhu YY, Wang Q (2015) Binding interaction of sorafenib with bovine serum albumin: spectroscopic methodologies and molecular docking. Spectrochim Acta A Mol Biomol Spectrosc 149:630–637
Jiao L, Li Y, Zhang Y, Liu J, Xie J, Zhang K, Zhou A (2017) Degradation kinetics of 6‴-p-coumaroylspinosin and identification of its metabolites by rat intestinal flora. J Agric Food Chem 65(22):4449–4455
Li Q, Du CH, Zhang M, Yan Y, Gao Y, Qin XM (2017) Investigation of effective components screening of Ziziphi Spinosae Semen based on serum pharmacochemistry and network pharmacology. Chin Tradit Herb Drugs 52(02):283–290
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81403130), Shanxi Scholarship Council of China (No. 2017-021) and Natural Science Foundation of Shanxi Province (No. 201801D121290).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors claim that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 508 kb)
Rights and permissions
About this article
Cite this article
Wu, B., Qu, C., Wang, Y. et al. Comparison of the Quenching Effects of Two Main Components of Ziziphi Spinosae Semen on Serum Albumin Fluorescence. J Fluoresc 29, 1113–1123 (2019). https://doi.org/10.1007/s10895-019-02422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02422-z