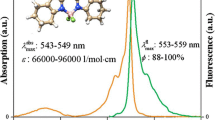
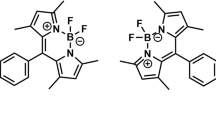
Abstract
Several boron-dipyrrin (BODIPY) based fluorophores with two and three dipyrrin cores were synthesized and investigated in solvents under the concentration variation. Comparative analysis of spectral and photophysical changes under increasing the number of the cores in the dye molecule was made. Mutual influence of dipyrrin cores was detected leading to the increasing of the compounds rigidity and, thus, the absence of fluorescent molecular rotor effects under the viscosity variation. Aggregation induced quenching, which was observed for many mono-domain BODIPY dyes is reduced in case of investigated poly-domain compounds.







Similar content being viewed by others
Abbreviations
- ASE:
-
Amplified spontaneous emission
- EAS:
-
Electronic absorption spectra
- FRET:
-
Fluorescence resonance energy transfer
- PAI-1:
-
Plasminogen activator type 1
- ROS:
-
Reactive oxygen species
References
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 107:4891–4932. https://doi.org/10.1021/cr078381n
Ulrich G, Ziessel R, Harriman A (2008) The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed 47:1184–1201. https://doi.org/10.1002/anie.200702070
Marfin YS, Merkushev DA, Levshanov GA, Rumyantsev EV (2014) Fluorescent properties of 8-phenylbodipy in ethanol - ethylene glycol mixed solutions. J Fluoresc 24:1613–1619. https://doi.org/10.1007/s10895-014-1447-3
Marfin YS, Solomonov AV, Timin AS, Rumyantsev EV (2017) Recent advances of individual BODIPY and BODIPY-based functional materials in medical diagnostics and treatment. Curr Med Chem 24:2745–2772. https://doi.org/10.2174/0929867324666170601092327
Marfin YS, Merkushev DA, Usoltsev SD, Shipalova MV, Rumyantsev EV (2015) Fluorescent properties of 8-substituted BODIPY dyes: influence of solvent effects. J Fluoresc 25:1517–1526. https://doi.org/10.1007/s10895-015-1643-9
Vodyanova OS, Kochergin BA, Usoltsev SD, Marfin YS, Rumyantsev EV, Aleksakhina EL, Tomilova IK (2017) BODIPY dyes in bio environment: spectral characteristics and possibilities for practical application. J Photochem Photobiol A Chem 350:44–51. https://doi.org/10.1016/j.jphotochem.2017.09.049.
Marfin YS, Kazak AV, Usoltsev S, Glukhovskoy ЕG (2017) Synthesis and spectral properties of preorganized BODIPYs in solutions and Langmuir-Schaefer films. Appl Surf Sci 424:228–238. https://doi.org/10.1016/J.APSUSC.2017.04.014
Marfin YS, Aleksakhina EL, Merkushev DA, Rumyantsev EV, Tomilova IK (2016) Interaction of BODIPY dyes with the blood plasma proteins. J Fluoresc 26:255–261. https://doi.org/10.1007/s10895-015-1707-x
Marfin YS, Shipalova MV, Kurzin VO, Ksenofontova KV, Solomonov AV, Rumyantsev EV (2016) Fluorescent properties of BODIPY sensors based on Photoinduced Electron transfer. J Fluoresc 26:2105–2112. https://doi.org/10.1007/s10895-016-1905-1
Marfin YS, Vodyanova OS, Merkushev DA, Usoltsev SD, Kurzin VO, Rumyantsev EV (2016) Effect of π-extended substituents on Photophysical properties of BODIPY dyes in solutions. J Fluoresc 26:1975–1985. https://doi.org/10.1007/s10895-016-1891-3
Aleksakhina EL, Marfin YS, Merkushev DA, Tomilova IK, Rumyantsev EV, Румянцев ЕВ, Tomilova IK, Константиновна ТИ, Rumyantsev EV, Владимирович РЕ (2015) Studying the blood clotting investigation in Prescence of boron-Dipyrrin fluorescent dyes. Kazan Meditsinskiy Zhurnal 96:792–798. https://doi.org/10.17750/KMJ2015-792
Kursunlu AN (2015) Synthesis and photophysical properties of modifiable single, dual, and triple-boron dipyrromethene (Bodipy) complexes. Tetrahedron Lett 56:1873–1877. https://doi.org/10.1016/j.tetlet.2015.02.097
Mirloup A, Retailleau P, Ziessel R (2013) Luminescent molecular solar concentrators made of multi-Bodipy dyes. Tetrahedron Lett 54:4456–4462. https://doi.org/10.1016/j.tetlet.2013.06.039
Ishizaki A, Calhoun TR, Schlau-Cohen GS, Fleming GR (2010) Quantum coherence and its interplay with protein environments in photosynthetic electronic energy transfer. Phys Chem Chem Phys 12:7319–7337. https://doi.org/10.1039/c003389h
Benniston AC, Copley G (2009) Lighting the way ahead with boron dipyrromethene (Bodipy) dyes. Phys Chem Chem Phys 11:4124–4131. https://doi.org/10.1039/b901383k
Bahaidarah E, Harriman A, Stachelek P, Rihn S, Heyer E, Ziessel R (2014) Fluorescent molecular rotors based on the BODIPY motif: effect of remote substituents. Photochem Photobiol Sci 13:1397–1401. https://doi.org/10.1039/c4pp00204k
Raut S, Kimball JD, Fudala R, Bora I, Chib R, Jaafari H, Castillo M, Smith NW, Dzyuba SV, Gryczynski Z (2016) Triazine-based BODIPY trimer as a molecular viscometer. Phys Chem Chem Phys 18:4535–4540. https://doi.org/10.1039/C5CP07214J.
Yang Y, Zhang L, Li B, Zhang L, Liu X (2013) Triphenylamine-cored tetramethyl-BODIPY dyes: synthesis, photophysics and lasing properties in organic media. RSC Adv 3:14993. https://doi.org/10.1039/c3ra42276c
Alamiry MAH, Benniston AC, Copley G, Elliott KJ, Harriman A, Stewart B, Zhi Y-G (2008) A molecular rotor based on an unhindered boron Dipyrromethene (Bodipy) dye. Chem Mater 20:4024–4032. https://doi.org/10.1021/cm800702c
Nepomnyashchii AB, Bröring M, Ahrens J, Bard AJ (2011) Synthesis, photophysical, electrochemical, and electrogenerated chemiluminescence studies. Multiple sequential electron transfers in BODIPY monomers, dimers, trimers, and polymer. J Am Chem Soc 133:8633–8645. https://doi.org/10.1021/ja2010219.
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172. https://doi.org/10.1039/C1CS15132K
Coskun A, Akkaya EU (2005) Ion Sensing Coupled to Resonance Energy Transfer: A Highly Selective and Sensitive Ratiometric Fluorescent Chemosensor for Ag(I) by a Modular Approach. J Am Chem Soc. https://doi.org/10.1021/JA052574F
Bergström F, Mikhalyov I, Hägglöf P, Wortmann R, Ny T, Johansson LBÅ (2002) Dimers of dipyrrometheneboron difluoride (BODIPY) with light spectroscopic applications in chemistry and biology. J Am Chem Soc 124:196–204. https://doi.org/10.1021/ja010983f
Kumaresan D, Agarwal N, Gupta I, Ravikanth M (2002) Synthesis of 21-thia and 21-oxaporphyrin building blocks and boron–dipyrrin appended systems. Tetrahedron 58:5347–5356. https://doi.org/10.1016/S0040-4020(02)00482-9
Azov VA, Schlegel A, Diederich F (2005) Geometrically precisely defined multinanometer expansion/contraction motions in a Resorcin[4]arene Cavitand based molecular switch. Angew Chem Int Ed 44:4635–4638. https://doi.org/10.1002/anie.200500970
Riddick JA, Bunger WB, Sakano TK (1986) Organic solvents: physical properties and methods of purification. Techniques of Chemistry, 4th edn. Wiley Intercedence, Hoboken
Margreth M, Schlink R, Steinbach A, Margreth M, Schlink R, Steinbach A (2010) Water determination by karl fischer titration. Pharm Sci Encycl John Wiley & Sons, Inc., Hoboken. https://doi.org/10.1002/9780470571224.pse415
Merkushev DA, Usoltsev SD, Marfin YS, Pushkarev AP, Volyniuk D, Grazulevicius JV, Rumyantsev EV (2017) BODIPY associates in organic matrices: spectral properties, photostability and evaluation as OLED emitters. Mater Chem Phys 187:104–111. https://doi.org/10.1016/j.matchemphys.2016.11.053
Castillo M, Raut SL, Price S, Bora I, Jameson LP, Qiu C, Schug KA, Gryczynski Z, Dzyuba SV (2016) Spectroscopic differentiation between monomeric and aggregated forms of BODIPY dyes: effect of 1,1-dichloroethane. RSC Adv 6:68705–68708. https://doi.org/10.1039/C6RA10833D
Vu TT, Dvorko M, Schmidt EY, Audibert JF, Retailleau P, Trofimov BA, Pansu RB, Clavier G, Méallet-Renault R (2013) Understanding the spectroscopic properties and aggregation process of a new emitting boron dipyrromethene (BODIPY). J Phys Chem C 117:5373–5385. https://doi.org/10.1021/jp3097555
Ma X, Sun R, Cheng J, Liu J, Gou F, Xiang H, Zhou X (2016) Fluorescence aggregation-caused quenching versus aggregation-induced emission: a visual teaching Technology for Undergraduate Chemistry Students. J Chem Educ 93:345–350. https://doi.org/10.1021/acs.jchemed.5b00483
Tleugabulova D, Zhang Z, Brennan JD (2002) Characterization of bodipy dimers formed in a molecularly confined environment. J Phys Chem B 106:13133–13138. https://doi.org/10.1021/jp027126y
Funding
The work was carried out with the financial support of the grant of the Russian Science Foundation No. 17–73-10408.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Several BODIPY-based fluorescent dyes were synthesized and characterized.
• The photophysical properties of poly-domain BODIPY dyes have been systematically investigated.
• Formation of J-type packing in the aggregated phase of BODIPY derivatives was observed.
Rights and permissions
About this article
Cite this article
Banakova, E., Marfin, Y., Molchanov, E. et al. Synthesis and Spectral Characteristics of BODIPY Dyes with Two or Three Dipyrrin Domains. J Fluoresc 29, 41–51 (2019). https://doi.org/10.1007/s10895-018-2308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2308-2