Abstract
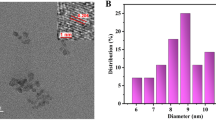
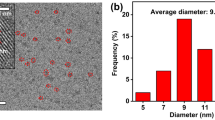
A green and simple microwave-assisted method was used to synthesis water-soluble boron and nitrogen-co-doped carbon dots (B-N-CDs). These B-N-CDs were successfully used for the fluorescent determination of Sn4+ and Mo6+ ions. This probe had a fast response time at pH = 4 with high sensitivity and selectivity. Linear correlation between F0/F and the concentration was seen in the range of 0.2–18 μM and 0.2–25 μM for Sn4+ and Mo6+, respectively. Under optimum condition, the limit of detection (LOD) for Sn4+ and Mo6+ were 150 nM and 132 nM, respectively. The performance of the sensor was evaluated by different real samples such as tap, river and mineral water, canned fish sample and tomato samples.





Similar content being viewed by others
References
Hubert C, Ziémons E, Rozet E, Breuer A, Lambert A, Jasselette C, De Bleye C, Lejeune R, Hubert P (2010) Development and validation of a quantitative method for the selective determination of tin species in tin octoate by differential pulse polarography. Talanta 80:1413–1420
Pérez-Herranz V, Garcia-Gabaldón M, Guiñón JL, Garcia-Antón J (2003) Effect of citric acid and hydrochloric acid on the polarographic behaviour of tin application to the determination of tin(II) in presence of tin(IV) in the activating solutions of the electrode less plating of polymers. Anal Chim Acta 484:243–251
Frena M, Campestrini I, de Braga OC, Spinelli A (2011) In situ bismuth-film electrode for square-wave anodic stripping voltammetric determination of tin in biodiesel. Electrochim Acta 56:4678–4684
Chomisteková Z, Culková E, Vojtko J, Brescher R, Tomčík P (2015) Voltammetric behavior of I2/2I− redox system on boron-doped diamond electrode in various media and its utilization for the indirect detection of tin(II). J Electroanal Chem 758:46–53
Gholivand MB, Babakhanian A, Rafiee E (2008) Determination of Sn(II) and Sn(IV) after mixed micelle-mediated cloud point extraction using polyoxometalate as a complexing agent by flame atomic absorption spectrometry. Talanta 76:503–508
Uluozlu OD, Tuzen M (2015) Carrier element-free coprecipitation and speciation of inorganic tin in beverage samples and total tin in food samples using N-benzoyl-N,Ndiisobutylthiourea and its determination by graphite furnace atomic absorption spectrometry. LWT Food Sci Technol 63:1091–1096
Prusa L, Dedina J, Kratzer J (2013) Ultratrace determination of tin by hydride generation in-atomizer trapping atomic absorption spectrometry. Anal Chim Acta 804:50–58
Galazzi RM, Arruda MAZ (2013) Optimization of a hydride generation metallic furnace atomic absorption spectrometry (HG-MF-AAS) method for tin determination: analytical and morphological parameters of a metallic atomizer. Talanta 117:543–548
Wolf SF, Compton JR, Gagnon CJL (2012) Determination of 11 major and minor elements in chondritic meteorites by inductively coupled plasma mass spectrometry. Talanta 100:276–281
Carricondo J, Iofrida M, Ararat C, Iribarren M, Corvalán C (2015) Laser induced–breakdown spectroscopy for quantitative Sn analysis in Zr based alloys. Procedia Mater Sci 9:129–134
Jiang X, Gan W, Wan L, Deng Y, Yang Q, He Y (2010) Electrochemical hydride generation atomic fluorescence spectrometry for detection of tin in canned foods using polyaniline-modified lead cathode. J Hazard Mater 184:331–336
Figi R, Nagel O, Tuchschmid M, Lienemann P, Gfeller U, Bukowiecki N (2010) Quantitative analysis of heavy metals in automotive brake linings: a comparison between wet-chemistry based analysis and in-situ screening with a handheld X-ray fluorescence spectrometer. Anal Chim Acta 676:46–52
Jiaming L, Guohui Z, Tianlong Y, Aihong W, Yan F, Longdi L (2003) Determination of trace tin by solid substrate-room temperature phosphorimetry using sodium dodecyl sulfate as sensitizer. Spectrochim Acta Part A 59:2081–2085
Boa Morte ES, Grac M, Korn A, Saraiva MLMFS, Lima JLFC, Pinto PCAG (2009) Sequential injection fluorimetric determination of Sn in juices of canned fruits. Talanta 79:1100–1103
Zhu L, Yang J, Wang Q, Zeng L (2014) Highly selective fluorescent probe for the detection of tin(IV) ion. J Lumin 148:161–164
Manzoori JL, Amjadi M, Abolhasani D (2006) Spectrofluorimetric determination of tin in canned foods. J Hazard Mater 137:1631–1635
Yazid SNAM, Chin SF, Pang SC, Ng SM (2013) Detection of Sn(II) ions via quenching of the fluorescence of carbon nanodots. Microchim Acta 180:137–143
Das AK, Chakraborty R, Cervera ML, Guardia M (2007) A review on molybdenum determination in solid geological samples. Talanta 71:987–1000
Gurkan R, Aksoy U, Ulusoy HI, Akcay M (2013) Determination of low levels of molybdenum (VI) in food samples and beverages by cloud point extraction coupled with flame atomic absorption spectrometry. J Food Compos Anal 32:74–82
Agrawal YK, Sharma KR (2005) Speciation, liquid–liquid extraction, sequential separation, preconcentration, transport and ICP-AES determination of Cr(III), Mo(VI) and W(VI) with calix-crown hydroxamic acid in high purity grade materials and environmental samples. Talanta 67:112–120
Reid HJ, Bashammakh AA, Goodall PS, Landon MR, O'Connor C, Sharp BL (2008) Determination of iodine and molybdenum in milk by quadrupole ICPMS. Talanta 75:189–197
Jiang C, Wang J, He F (2001) Spectrofluorimetric determination of trace amounts of molybdenum in pig liver and mussels. Anal Chim Acta 439:307–313
Capitan F, Sanchez-Palencia G, Naval A, Capitan-Vallvey LF, Vilchez JL (1992) Simultaneous determination of molybdenum and tungsten by first-derivative synchronous solid-phase spectrofluorimetry. Anal Chim Acta 259:345–353
Blanco CC, Campana AG, Barrero FA, Ceba MR (1993) Simultaneous spectrofluorimetric determination of traces of molybdenum and boron in plant leaves. Anal Chim Acta 283:213–223
Kawakubo S, Suzuki H, Iwatsukic M (1996) Catalytic spectrofluorometric determination of ultratrace molybdenum in natural fresh water. Anal Sci 12:767–771
Blanco CC, Campana AG, Barrero FA, Ceba MR (1995) Micellar medium for the analysis of complex mixtures of molybdenum and tungsten by derivative synchronous spectrofluorimetry in steels. Talanta 42:1037–1044
Ensafi AA, Khaloo SS (2005) Determination of traces molybdenum by catalytic adsorptive stripping voltammetry. Talanta 65:781–788
Deng P, Fei J, Zhang J, Feng Y (2011) Determination of molybdenum by adsorptive anodic stripping voltammetry of molybdenum–alizarin violet complex at an acetylene black paste electrode. Food Chem 124:1231–1237
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49:6726–6744
Wang Y, Kim SH, Feng L (2015) Highly luminescent N, S-co-doped carbon dots and their direct use as mercury (II) sensor. Anal Chim Acta 890:134–142
Bourlinos AB, Trivizas G, Karakassides MA, Baikousi M, Kouloumpis A, Gournis D, Bakandritsos A, Hola K, Kazak O, Zboril R, Papagiannouli I, Aloukos P, Couris S (2015) Green and simple route toward boron doped carbon dots with significantly enhanced non-linear optical properties. Carbon 83:173–179
Lesková M, Sklenárová H, Bazel Y, Chocholous P, Solich P, Andruch V (2012) A non-extractive sequential injection method for determination of molybdenum. Talanta 96:185–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabaraki, R., Abdi, O. & Yousefipour, S. Green and Selective Fluorescent Sensor for Detection of Sn (IV) and Mo (VI) Based on Boron and Nitrogen-Co-Doped Carbon Dots. J Fluoresc 27, 651–657 (2017). https://doi.org/10.1007/s10895-016-1994-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1994-x