Abstract
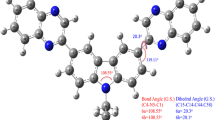
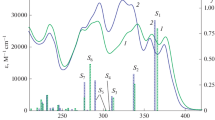
Structural influence on the photophysical behavior of two pairs of molecular systems from the biologically potent benzoquinoline family, namely, dimethyl-3-(4-chlorophenyl)-3,4-dihydrobenzo[f]-quinoline-1,2-dicarboxylate, dimethyl-3-(2,6-dichlorophenyl)-3,4-dihydrobenzo[f]quinoline-1,2-dicarboxylate and their corresponding dehydrogenated analogues has been investigated exploiting experimental as well as computational techniques. The study unveils that dehydrogenation in the heterocyclic rings of the studied quinoline derivatives modifies their photophysics radically. Experimental observations imply that the photophysical behavior of the dihydro analogues is governed by the intramolecular charge transfer (ICT) process. However, the ICT process is restricted significantly by the dehydrogenation of the heterocyclic rings. Computational exertion leads to the proposition that the change in the electronic distribution in these molecular systems on dehydrogenation is the rationale behind the dramatic modification of their photophysics.






Similar content being viewed by others
References
Law K-Y (1987) Squaraine chemistry: effects of structural changes on the absorption and multiple fluorescence emission of bis[4-(dimethylamino)phenyl]squaraine and its derivatives. J Phys Chem 91:5184–5193
Allen NS, Mckellar JF (1977) Structural influences on the fluorescence of aminoanthraquinones. J Photochem 7:107–111
Cowgill RW (1967) Fluorescence and protein structure: X. Reappraisal of solvent and structural effects. Biochim Biophys Acta- Protein Structure 133:6–18
Neves P, Leite A, Rangel M, de Castro B, Gameiro P (2007) Influence of structural factors on the enhanced activity of moxifloxacin: a fluorescence and EPR spectroscopic study. Anal Bioanal Chem 387:1543–1552
Tu O, Knott T, Marsh M, Bechtol K, Harris D, Barker D, Bashkin J (1998) The influence of fluorescent dye structure on the electrophoretic mobility of end-labeled DNA. Nucl Acids Res 26:2797–2802
Sarkar D, Das P, Basak S, Chattopadhyay N (2008) Binding interaction of cationic phenazinium dyes with calf thymus DNA: a comparative study. J Phys Chem B 112:9243–9249
Seeman P, Guan HC, Nobrega J, Jiwa D, Markstein R, Balk JH, Picetti R, Borrelli E, Van Tol HH (1997) Dopamine D2-like sites in schizophrenia, but not in Alzheimer's, Huntington's, or control brains, for [3 H]benzquinoline. Synapse 25:137–146
Schmittel M, Ammon H (1998) A short synthetic route to 4,7-dihalogenated 1,10-phenanthrolines with additional groups in 3,8-position: soluble precursors for macrocyclic oligophenanthrolines. Eur J Org Chem 1998:785–792
Gladiali S, Chelucci G, Mudadu MS, Gastaut MA, Thummel RP (2001) Friedländer synthesis of chiral alkyl-substituted 1,10-phenanthrolines. J Org Chem 66:400–405
Selvi G, Rajendran SP (2004) Synthesis of some new 2-[3-(2-chloroquinolinyl)]-3-aryl-4-thiazolidinones as potent antibacterial agents. J Asian Chem 16:1017–1022
Bahuguna RP, Joshi BC (1994) Synthesis and antibacterial activity of some novel substituted arylsulfonylbenzo[f]quinolines. Ind J Heterocycl Chem 3:265–268
Szmuszkovicz J, Darlington WH, Von Voigtlander PF (1988) Preparation and formulation of antipsychotic aminopolyhydrobenz(iso)quinolines and intermediates. Chem Abstr 110:75335
Nozulak J, Vigouret JM, Jaton AL, Hofmann A, Dravid AR, Weber HP, Kalkman HO, Walkinshaw MD (1992) Centrally acting α1-adrenoceptor agonists based on hexahydronaphth[2,3-b]-1,4-oxazines and octahydrobenzo[g]quinolines. J Med Chem 35:480–489
Mikhailitsyn FS, Kozyreva NP, Rabinovich SA, Maksakovskaya YV, Kulikovskaya IM, Dadasheva NR, Lebedeva MN, Bekhli AF, Lychko ND, Uvarova NA (1992) The search for new antiparasitic agents. 10. The synthesis, toxicological and antimalarial properties of nitrogen-containing heterocycles with a 4-(4-alkylpiperazinyl-1) phenylamine substituent (the preparation quinoprazine). Med Parazitol Parazit Bolezni 50–53 Chem Abstr 117: 251317
Bahuguna RP, Joshi BC, Mangal HN (1992) Studies on benzoquinoline derivatives: preparation and antimicrobial activity of azo-derivatives of arylthiobenzo[f]quinoline. J Ind Chem Soc 69:401–402
Carr BA, Franklin MR (1998) Drug-metabolizing enzyme induction by 2,2'-dipyridyl, 1,7-phenanthroline, 7,8-benzoquinoline and oltipraz in mouse. Xenobiotica 28:949–956
Le HT, Lamb JG, Franklin MR (1996) Drug metabolizing enzyme induction by benzoquinolines, acridine, and quinacrine; tricyclic aromatic molecules containing a single heterocyclic nitrogen. J Biochem Toxicol 11:297–303
Mataga N (1984) Photochemical charge transfer phenomena — picosecond laser photolysis studies. Pure Appl Chem 56:1255–1268
Barbara F, Jarzeba W (1990) Ultrafast photochemical intramolecular charge transfer and excited state salvation. Adv Photochem 15:1–68
Verhoeven JW (1990) Electron transport via saturated hydrocarbon bridges: 'exciplex' emission from flexible, rigid and semiflexible bichromophores. Pure Appl Chem 62:1585–1596
Kakitani T, Matsuda N, Yoshimori A, Mataga N (1995) Present and future perspectives of theoretical aspects of photoinduced charge separation and charge recombination reactions in solution. Prog React Kinet 20:347–381
Mataga N, Miyasaka H (1999) Electron transfer and exciplex chemistry. Adv Chem Phys 107:431–496
Marcus RA (1990) Reorganization free energy for eiectron transfers at liquid-liquid and dielectric semiconductor-liquid interfaces. J Phys Chem 94:1050–1055
Lai RY, Fabrizio EF, Lu L, Jenekhe SA, Bard AJ (2001) Synthesis, cyclic voltammetric studies, and electrogenerated chemiluminescence of a new donorsacceptor molecule: 3,7-[bis[4-phenyl-2-quinolyl]]-10-methylphenothiazine. J Am Chem Soc 123:9112–9118
Sun XB, Liu YQ, Xu XJ, Yang CH, Yu G, Chen SY, Zhao HZ, Qiu FW, Li YF, Zhu DB (2005) Novel electroactive and photoactive molecular materials based on conjugated donor-acceptor structures for optoelectronic device applications. J Phys Chem B 109:10786–10792
He C, He Q, He Y, Li Y, Bai F, Yang C, Ding Y, Wang L, Ye J (2006) Organic solar cells based on the spin-coated blend films of TPA-th-TPA and PCBM. Solar Energy Mater Solar Cells 90:1815–1827
Kim YH, Cho DW, Yoon M, Kim D (1996) Observation of hydrogen-bonding effects on twisted intramolecular charge transfer of p-(N,N-diethylamino)benzoic acid in aqueous cyclodextrin solutions. J Phys Chem 100:15670–15676
Mallick A, Haldar B, Chattopadhyay N (2005) Spectroscopic investigation on the interaction of ICT probe 3-acetyl-4-oxo-6,7-dihydro-12 H indolo-[2,3-a] quinolizine with serum albumins. J Phys Chem B 109:14683–14690
Maiti G, Karmakar R, Kayal U (2013) One pot imino Diels–Alder reaction for the synthesis of 3-aryl-3,4-dihydro- benzo[f]quinoline derivatives catalyzed by antimony trichloride. Tetrahedron Lett 54:2920–2923
Jones G II, Jackson WR, Choi C-Y, Bergmark WR (1985) Solvent effects on emission yleld and llfetime for coumarin laser dyes. Requirements for a rotatory decay mechanism. J Phys Chem 89:294–300
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven TJ, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2009) Gaussian 09. Gaussian, Inc., CT, Wallingford
Mishra H, Maheshwary S, Tripathi HB, Satyamurthy N (2005) An experimental and theoretical investigation of the photophysics of 1-hydroxy-2-naphthoic acid. J Phys Chem A 109:2746–2754
Catalan J, Valle JC, Palomar J, Diaz C, Paz JLG (1999) The six-membered intramolecular hydrogen bond position as a switch for inducing an excited state intramolecular proton transfer (ESIPT) in esters of o-hydroxynaphthoic acids. J Phys Chem A 103:10921–10934
De SP, Ash S, Dalai S, Misra A (2007) A DFT-based comparative study on the excited states intramolecular proton transfer in 1-hydroxy-2-naphthaldehyde and 2-hydroxy-3-naphthaldehyde. J Mol Struct THEOCHEM 807:33–41
Paul BK, Mahanta S, Singh RB, Guchhait N (2010) A DFT-based theoretical study on the photophysics of 4-hydroxyacridine: single-water-mediated excited state proton transfer. J Phys Chem A 114:2618–2627
Becke AD (1993) Density-functional thermochemistry. III The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Takano Y, Houk KN (2005) Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous salvation free energies of neutral and ionic organic molecules. J Chem Theory Comput 1:70–77
Purkayastha P, Chattopadhyay N (2000) Role of rotamerization and excited state intramolecular proton transfer in the photophysics of 2-(2′-hydroxyphenyl) benzoxazole, 2-(2′-hydroxyphenyl)benzimidazole and 2-(2′-hydroxyphenyl) benzthiazole: a theoretical study. Phys Chem Chem Phys 2:203–210
Purkayastha P, Chattopadhyay N (2003) Theoretical modeling for the ground state rotamerisation and excited state intramolecular proton transfer of 2-(2'-hydroxyphenyl)oxazole, 2-(2'-hydroxyphenyl)imidazole, 2-(2'-hydroxyphenyl)thiazole and their benzo analogues. Int J Mol Sci 4:335–361
Mallick A, Maiti S, Haldar B, Purkayastha P, Chattopadhyay N (2003) Photophysics of 3-acetyl-4-oxo-6,7-dihydro-12 H indolo-[2,3-a] quinolizine: emission from two states. Chem Phys Lett 371:688–693
Cichos F, Willert A, Rempel U, Borczyskowski CV (1997) Solvation dynamics in mixtures of polar and nonpolar solvents. J Phys Chem A 101:8179–8185
Kosower EM (1982) Intramolecular donor-acceptor systems. 9. Photophysics of (Pheny1amino)naphthalenesulfonates: a paradigm for excited-state intramolecular charge transfer. Acc Chem Res 15:259–266
Kosower EM, Tanizawa K (1972) Analysis of fluorescence emission and quenching for molecules bearing latent donors. Chem Phys Lett 16:419–425
Kosower EM, Dodiuk H, Tanizawa K, Ottolenghi M, Orbach N (1975) Intramolecular donor-acceptor systems. Radiative and nonradiative processes for the excited states of 2-N-Arylamino-6-naphthalenesulfonates. J Am Chem Soc 97:2167–2178
Piet JJ, Schuddeboom W, Wegewijs BR, Grozema FC, Warman JM (2001) Symmetry breaking in the relaxed S1 excited state of bianthryl derivatives in weakly polar solvents. J Am Chem Soc 123:5337–5347
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Prendergast FG (1991) Time-resolved fluorescence techniques: method and application in biology. Curr Opin Struct Biol 1:1054–1059
Chattopadhyay A, Mukherjee S, Raghuraman H (2002) Reverse micellar organization and dynamics: a wavelength-selective fluorescence approach. J Phys Chem B 106:13002–13009
Bhattacharyya K, Chowdhury M (1993) Environmental and magnetic field effects on exciplex and twisted charge transfer emission. Chem Rev 93:507–535
Kundu S, Bera SC, Chattopadhyay N (1998) Twisted intramolecular charge transfer of DMABA in cyclodextrin environments. Ind J Chem A 37:102–108
Acknowledgments
Financial support from the Council of Scientific and Industrial Research, Government of India (Project No. 01/(2807)/14/EMR-II), is gratefully acknowledged. P.K. and S.G. thank Council of Scientific and Industrial Research and University Grants Commission respectively for their research fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Structural impact on the photophysics of synthesized benzoquinolines is studied• Drastic modification in the photophysics results upon dehydrogenation• Dihydrobenzoquinolines give dual emissions for the normal and the ICT species• The ICT species is formed in the ground as well as in the photoexcited states • Dehydrogenation in the heterocyclic ring leads to suppression of the ICT emission
Electronic Supplementary Material
ESM 1
(DOC 3069 kb)
Rights and permissions
About this article
Cite this article
Kundu, P., Ghosh, S., Karmakar, R. et al. Impact of Structural Modification on the Photophysical Response of Benzoquinoline Fluorophores. J Fluoresc 26, 845–854 (2016). https://doi.org/10.1007/s10895-016-1772-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1772-9