Abstract
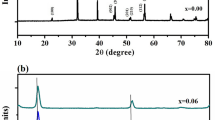
Pure and 0.5 to 10 mole% Sm3+ doped TTB (tetragonal tungsten bronze)-type BaTa2O6 ceramic phosphor was produced by the solid state reaction method which performed at 1425 °C for 20 h. XRD and SEM analysis indicated single TTB phase for undoped and 0.5 to 10 mole% Sm3+ doped BaTa2O6 structures. SEM also showed that the BaTa2O6 grain size decreased with the increasing content of Sm3+. Optical analysis indicated significant emissions in the visible spectral region as green (λ = 562.7 nm) and orange-reddish (λ = 597.1 nm). The emission intensity increased with the increasing doping concentration up to 2.5 mole%, and then decreased due to the concentration quenching effect.









Similar content being viewed by others
References
Castro Y, Julian B, Boissiére C, Viana B, Amenitsch H, Grosso D, Sanchez C (2007) Synthesis, characterization and optical properties of Eu2O3 mesoporous thin films. Nanotechnology 18:55705
Wang D, Yin QR, Li YX, Wang MQ (2002) Concentration quenching of Eu2+ in SrO · Al2O3:Eu2+ phosphor. J Lumin 97:1–6
Chang H, Lenggoro IW, Ogi T, Okuyama K (2005) Direct synthesis of barium magnesium aluminate blue phosphor particles via a flame route. Mater Lett 59:1183
Yang HM, Shi JX, Gong ML (2005) A novel red emitting phosphor Ca2SnO4: Eu3+”. J Solid State Chem 178:917–920
Fang TH, Hsiao YJ, Chang YS, Chang YH (2006) Photoluminescent characterization of KNbO3: Eu3+. Mater Chem Phys 100:418–422
Welker T (1991) Recent developments on phosphors for fluorescent lamps and cathode-ray tubes. J Lumin 48–49:49–56
Vedda A, Martini M, Nikl M, Mihokova E, Nitsch K, Solovieva N, Karagulian F (2002) Optical absorption and thermoluminescence of Tb3+-doped phosphate scintillating glasses. J Phys Condens Matter 14:7417
Nakamura S, Fasol G (1997) The blue laser diode: GaN based light emitters and laser. Springer, Berlin
Levine AK, Palilla FC (1994) A new, highly efficient red-emitting cathodoluminescent phosphor (YVO4:Eu) for color television. Appl Phys Lett 5:5118–5120
Rao RP (2005) Tm3+ activated lanthanum phosphate: a blue PDP phosphor. J Lumin 113:271–278
Yan ZG, Yan CH (2008) Controlled synthesis of rare earth nanostructures. J Mater Chem 18:5046–5059
Magneli A (1949) Ark Kemi 1:213–221
Simon A, Ravez J (2006) Solid-state chemistry and non-linear tungsten bronzes materials. C R Chim 9:1268–1276
Roulland F, Josse M, Castel E, Maglione M (2009) Influence of ceramic process and Eu content on the composite multiferroic properties of the Ba6-2x Ln2xFe1+xNb9-xO30 TTB system. Solid State Sci 1:1709–1716
Kovba LM, Lykova LN, Paromova MV, Lopato LM, Shevchenko AV (1977) Barium oxide-tantalum oxide system. Russ J Inorg Chem 22:1544
Layden GK (1968) Dielectric and structure studies of hexagonal BaTa2O6. Mater Res Bull 3:349
Smolenskii GA, Isupov VA, Agranovskaia AI (1956) Sov Phys 300:1
Ichinose N, Shimada T (2006) Effect of grain size and secondary phase on microwave dielectric properties of Ba(Mg1/3Ta2/3)O3 and Ba([Mg, Zn]1/3Ta2/3)O3 systems. J Eur Ceram Soc 26:1755–1759
Lee YH, Kim YS, Kim DH, Oh MH (2007) Conduction mechanisms in barium tantalates films and modification of interfacial barrier height. IEEE Trans Electron Devices 47:71–76
Kato H, Kudo A (1998) New tantalate photocatalysts for water decomposition into H2 and O2. Chem Phys Lett 295:487–492
Layden GK (1967) Polymorphism of BaTa2O6. Mater Res Bull 2:533
Vanderah TA, Roth RS, Siegrist T, Febo W, Loezos JM, Wong-Ng W (2003) Subsolidus phase equilibria and crystal chemistry in the system BaO–TiO2–Ta2O5. Solid State Sci 5:149–164
Mumme WG, Grey IE, Roth RS, Vanderah TA (2007) Contrasting oxide crystal chemistry of Nb and Ta: the structures of the hexagonal bronzes BaTa2O6 and Ba0.93Nb2.03O6. J Solid State Chem 180:2429–2436
Navale SC, Samuel V, Gaikwad AB, Ravi V (2007) A co-precipitation technique to prepare BaTa2O6. Ceram Int 33:297–299
İlhan M, Mergen A, Yaman C (2011) Mechanochemical synthesis and characterisation of BaTa2O6 ceramic powders. Ceram Int 37:1507–1514
İlhan M, Mergen A, Yaman C (2013) Removal of iron from BaTa2O6 ceramic powder produced by high energy milling. Ceram Int 39:5741–5750
Speight JG (1999) Lange’s handbook of chemistry, 16th edn. The McGraw-Hill Companies, New York
Erkmen EZ (2012) Malzeme Karakterizasyonu ve Temel İlkeleri. Yalın Yayıncılık, İstanbul
Garciá JS, Bausá LE, Jaque D (2005) An introduction to the optical spectroscopy of inorganic solids. Wiley, England
Pang TP, Yang MR, Chen KS (2000) Photoluminescence of ZnS:Sm phosphor prepared in a reductive atmosphere. Ceram Int 26:153–158
Kaur G, Dwivedi Y, Rai SB (2010) Study of enhanced red emission from Sm(Sal)3 Phen ternary complexes in Poly Vinly alcohol film. Opt Commun 283:3441–3447
Yerpude AN, Dhoble SJ (2012) Synthesis and photoluminescence properties of Dy3+, Sm3+ activated Sr5SiO4Cl6 phosphor. J Lumin 132:2975–2978
Acknowledgments
The authors would like to thanks to Marmara University Research Fund (BAPKO) for supporting this research. Project No. FEN-A-150513-0167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekmekçi, M.K., İlhan, M., Başak, A.S. et al. Structural and Luminescence Properties of Sm3+ Doped TTB -Type BaTa2O6 Ceramic Phosphors. J Fluoresc 25, 1757–1762 (2015). https://doi.org/10.1007/s10895-015-1663-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1663-5