Abstract
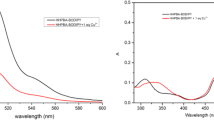
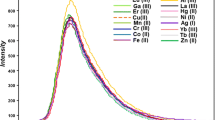
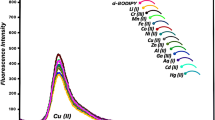
Copper is an essential trace element for the proper functioning of organ and metabolic process in humans. However, both its excess and deficiency in the body can result in adverse health effects. A BODIPY containing 2,2′-bipyridyl group was synthesized and used as a fluorescent chemodosimeter for selective Cu2+ detection in mild condition. This BODIPY shows fast response (~1 min) and high sensitivity for Cu2+ in aqueous solution due to the photoinduced electron transfer from the excited state of fluorophore to the bipyridyl unit complexed to Cu2+. The fluorescence quenching mechanism revealed by MALDI-TOF Mass spectra showed one Cu2+ could coordinate with two BODIPY molecules, and this coordination is reversible. This simple BODIPY dyes also could be used for sensing the Cu2+ in living cell. This work contributes to extend the potential applications of BODIPY to the biological and environmental areas.






Similar content being viewed by others
References
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S–811S
Georgopoulos PG, Roy A, Yonone-Lioy MJ, Opiekun RE, Lioy PJ (2001) Environmental copper: its dynamics and human exposure issues. J Toxicol Environ Health B Crit Rev 4:341–394
Suresh M, Ghosh A, Das A (2008) A simple chemosensor for Hg2+ and Cu2+ that works asa molecular keypad lock. Chem Commun 23(33):3906–3908
Royzen M, Dai Z, Canary JW (2005) Ratiometric displacement approach to Cu(II) sensing by fluorescence. J Am Chem Soc 127:1612–1613
Wang S, Men G, Zhao L, Hou Q, Jiang S (2010) Sensors Actuators A 145:826–831
Jiang J, Jiang H, Tang X, Yang L, Dou W, Liu W, Fang R, Liu W (2011) Dalton Trans 40:6367–6370
Yang Y, Huo F, Yin C, Chu Y, Chao J, Zhang Y, Zhang J, Li S, Lv H, Zheng A, Liu D (2013) Sensors Actuators B 177:1189–1197
Wu QY, Anslyn EV (2004) Catalytic signal amplification using a heck reaction. An example in the fluorescence sensing of Cu(II). J Am Chem Soc 126:14682–14683
Xu Z, Qian X, Cui J (2005) Ratiometric displacement approach to Cu(II) sensing by fluorescence. Org Lett 7:3029–3032
Weng YQ, Yue F, Zhong YR, Ye BH (2007) Inorg Chem 46:7749–7755
Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ (2006) A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc 128:10–11
Shao N, Zhang Y, Cheung S, Yang R, Chan W, Mo T, Li K, Liu F (2005) Copper ion-selective fluorescent sensor based on the inner filter effect using a spiropyran derivative. Anal Chem 77:7294–7303
Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ (2005) Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron X-ray fluorescence microscopy. Proc Natl Acad Sci U S A 102:11179–11184
Kramer R (1998) Fluoreszenz-Chemosensoren for Cu2+-Ionen: schnell, selektiv und hochempfindlich. Angew Chem 110:804–806
Zhang X, Shiraishi Y, Hirai T (2007) Cu(II)-selective green fluorescence of a rhodamine—diacetic acid conjugate. Org Lett 9:5039–5042
Xu ZC, Xiao Y, Qian XH, Cui JN, Cui DW (2005) Ratiometric and selective fluorescent sensor for CuII based on internal charge transfer (ICT). Org Lett 7:889–892
Kim MH, Jang HH, Yi S, Chang SK, Han MS (2009) Coumarin-derivative-based off-on catalytic chemodosimeter for Cu2+ ions. Chem Commun 4838–4840
Li N, Xiang Y, Tong A (2010) Highly sensitive and selective “turn-on” fluorescent chemodosimeter for Cu2+ in water via Cu2+-promoted hydrolysis of lactone moiety in coumarin. Chem Commun 46:3363–3365
Wen ZC, Yang R, He H, Jiang YB (2006) A highly selective charge transfer fluoroionophore for Cu2+. Chem Commun 106–108
Lee MH, Kim HJ, Yoon S, Park N, Kim JS (2008) Metal ion induced FRET OFF-ON in trendansyl-appended rhodamine. Org Lett 10:213–216
Xiang Y, Tong A, Jin P, Ju Y (2006) New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org Lett 8:2863–2866
Xiang Y, Li Z, Chen X, Tong A (2008) Highly sensitive and selective optical chemosensor for determination of Cu2+ in aqueous solution. Talanta 74:1148–1153
Shiraishi Y, Tanaka K, Hirai T (2013) Colorimetric sensing of Cu(II) in aqueous media with a spiropyran derivative via a oxidative dehydrogenation mechanism. ACS Appl Mater Interfaces 5:3456–3465
Yu MM, Li ZX, Wei LH, Wei DH, Tang MS (2008) A 1,8-naphthyridine-based fluorescent chemodosimeter for the rapid detection of Zn2+ and Cu2+. Org Lett 22:5115–5118
Jung HS, Kwon PS, Lee JW, Kim JI, Hong CS, Kim JW, Yan SH, Lee JY, Lee JH, Joo T, Kim JS (2009) Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108:4328–4359
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Fabbrizzi L, Poggi A (1995) Sensors and switches from supramolecular chemistry. Chem Soc Rev 24:197–202
Valeur B (2002) Molecular fluorescence. Principles and applications. Wiley-VCH, Weinheim
Baruah M, Qin W, Vallee RAL, Beljonne D, Rohand T, Dehaen W, Boens N (2005) A highly potassium-selective ratiometric fluorescent indicator based on MBDP azacrown ether excitable with visible light. Org Lett 7:4377–4380
Wang D, Shiraishi Y, Hirai T (2010) A distyryl MBDP derivative as a fluorescent probe for selective detection of chromium(III). Tetrahedron Lett 51:2545–2549
Coskun A, Deniz E, Akkaya EU (2007) A sensitive fluorescent chemosensor for anions based on a styryl-boradiazaindacene framework. Tetrahedron Lett 48:5359–5361
Werner T, Huber C, Heinl S, Kollmannsberger M, Daub J, Wolfbeis OS (1997) Novel optical pH-sensor based on a boradiaza-indacene derivative. Fresenius J Anal Chem 359:150–154
Kollmannsberger M, Gareis T, Heinl S, Breu J, Daub J (1997) Electrogenerated chemiluminescence and proton-dependent switching of fluorescence: functionalized difluoroboradiaza-s-indacenes. Angew Chem Int Ed Engl 36:1333–1335
Kim HJ, Kim JS (2006) BODIPY appended cone-calix[4]arene: selective fluorescence changes upon Ca2+ binding. Tetrahedron Lett 47:7051–7055
Sibrian-Vazquez M, Escobedo JO, Lowry M, Fronczek FR, Strongin RM (2012) Field effects induce bathochromic shifts in xanthene dyes. J Am Chem Soc 134:10502–10508
Zhu LN, Yang CL, Qin JG (2008) An aggregation-induced blue shift of emission and the self-assembly of nanoparticles from a novel amphiphilic oligofluorene. Chem Commun 6303–6305
Bodell WJ, Ye Q, Pathak DN, Pongracz K (1998) Oxidation of eugenol to form DNA adducts and 8-hydroxy-29-deoxyguanosine: role of quinone methide derivative in DNA adduct formation. Carcinogenesis 19:437–443
Stites TE, Mitchell AE, Rucker RB (2000) Physiological importance of quinoenzymes and the O-Quinone family of cofactors. J Nutr 130:719–727
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172
Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (Project No. 91227118) and China Postdoctoral Science Foundation (No. 2013M540260).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 996 kb)
Rights and permissions
About this article
Cite this article
Quan, L., Sun, T., Lin, W. et al. BODIPY Fluorescent Chemosensor for Cu2+ Detection and Its Applications in Living Cells: Fast Response and High Sensitivity. J Fluoresc 24, 841–846 (2014). https://doi.org/10.1007/s10895-014-1360-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1360-9