Abstract
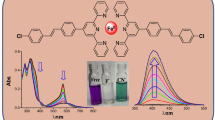
In this paper, anion binding and sensing affinity of the simple and easy-to-make salen, a typical class of ligand used comprehensively in metal coordination, was investigated. Results indicated that salophen was both a colorimetric and fluorescent selective chemosensor for fluoride ion, which operated by the anion-induced conformational changes and subsequently excited-state intramolecular proton transfer (ESPT) process. The F--induced quick response, as well as noticeable optical changes, suggested that anion-sensing mechanism maybe help to design and to synthesize the new preferential selective probes for F-.





Similar content being viewed by others
References
Yoon TP, Jacobsen EN (2003) Privileged chiral catalysts. Science 299:1691–1693. doi:10.1126/science.1083622
Zhang W, Loebach JL, Wilson SR, Jacobsen EN (1990) Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J Am Chem Soc 112:2801–2803. doi:10.1021/ja00163a052
Irie R, Noda K, Ito Y, Matsumoto N, Katsuki T (1990) Catalytic asymmetric epoxidation of unfunctionalized olefins. Tetrahedron Letters 31:7345–7348. doi:10.1016/s0040-4039(00)88562-7
Cozzi PG (2004) Metal-Salen Schiff base complexes in catalysis: practical aspects. Chem Soc Rev 33:410–421. doi:10.1039/b307853c
Haak RM, Wezenberg SJ, Kleij AW (2010) Cooperative multimetallic catalysis using metallosalens. Chem Commun 2713–2723. doi:10.1039/c001392g
Garnovskii AD, Nivorozhkin AL, Minkin VI (1993) Ligand environment and the structure of schiff base adducts and tetracoordinated metal-chelates. Coord Chem Rev 126:1–69. doi:10.1016/0010-8545(93)85032-y
Zhang C, Zhang X, Zhang X, Ou X, Zhang W, Jie J, Chang JC, Lee C-S, Lee S-T (2009) Facile one-step fabrication of ordered organic nanowire films. Adv Mater 21:4172–4175. doi:10.1002/adma.200802793
Wezenberg SJ, Kleij AW (2008) Material applications for salen frameworks. Angew Chem Int Ed 47:2354–2364. doi:10.1002/anie.200702468
Cho S-H, Ma B, Nguyen ST, Hupp JT, Albrecht-Schmitt TE (2006) A metal-organic framework material that functions as an enantioselective catalyst for olefin epoxidation. Chem Commun 2563–2565. doi:10.1039/b600408c
Routier SB, Bernier JL, Catteau MP, Bailly C (1997) Highly preferential cleavage of unpaired guanines in DNA by a functionalized salen-nickel complex. Bioorg Med Chem Lett 7:63–66. doi:10.1016/S0960-894X(96)00569-0
Puglisi A, Tabbi G, Vecchio G (2004) Bioconjugates of cyclodextrins of manganese salen-type ligand with superoxide dismutase activity. J Inorg Biochem 98:969–976. doi:10.1016/j.jinorgbio.2004.02.012
Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J, Malfroy B (2002) Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure − activity relationship studies. J Med Chem 45:4549–4558. doi:10.1021/jm020207y
Dalla CA, De Bernardin P, Forte G, Yafteh MF (2010) Metal-salophen-based receptors for anions. Chem Soc Rev 39:3863–3874. doi:10.1039/b926222a
Ganjali MR, Norouzi P, Hatambeygi N, Salavati-Niasari M (2006) Anion recognition: fabrication of a highly selective and sensitive HPO 2-4 PVC sensor based on a oxo-molybdenum methyl-salen. J Brazil Chem Soc 17:859–865
Mao S, Liu K, Lu F, Du L (2010) Colorimetric sensors based on hydrogen-bond-induced π-delocalization and/or anion-triggered deprotonation. Mini-Rev Org Chem 7:221–229
Arnendola V, Bonizzoni M, Esteban-Gomez D, Fabbrizzi L, Licchelli M, Sancenon F, Taglietti A (2006) Some guidelines for the design of anion receptors. Coord Chem Rev 250:1451–1470. doi:10.1016/j.ccr.2006.01.006
Rudkevich DM, Stauthamer WPRV, Verboom W, Engbersen JFJ, Harkema S, Reinhoudt DN (1992) UO2-salenes: neutral receptors for anions with a high selectivity for dihydrogen phosphate. J Am Chem Soc 114:9671–9673. doi:10.1021/ja00050a064
Vigato PA, Tamburini S (2004) The challenge of cyclic and acyclic schiff bases and related derivatives. Coord Chem Rev 248:1717–2128. doi:10.1016/j.cct.2003.09.003
Cametti M, Dalla Cort A, Mandolini L, Nissinen M, Rissanen K (2008) Specific recognition of fluoride anion using a metallamacrocycle incorporating a uranyl-salen unit. New J Chem 32:1113–1116. doi:10.1039/b806149a
Bandoli G, Clemente DA, Croatto JU, Vidali M, Vigato PA (1971) Preparation and crystal and molecular structure of [NN′-o-phenylene-bis(salicylideneiminato)UO2(EtOH)]. J Chem Soc D 1330–1331. doi:10.1039/C29710001330
Bandoli G, Clemente DA (1975) Preparation and crystal structure of aqua[bis(2-hydroxyphenylimino)-ethanato-OO′NN′-]dioxouranium. J Chem Soc, Dalton Trans: 612–615. doi:10.1039/DT9750000612
Cort AD, Mandolini L, Pasquini C, Rissanen K, Russo L, Schiaffno L (2007) Zinc-salophen complexes as selective receptors for tertiary amines. New J Chem 31:1633–1638. doi:10.1039/b700723j
Germain ME, Vargo TR, McClure BA, Rack JJ, Patten PGV, Odoi M, Knapp MJ (2008) Quenching mechanism of Zn(Salicylaldimine) by nitroaromatics. Inorg Chem 47:6203–6211. doi:10.1021/ic702469q
Kleij AW, Kuil M, Tooke DM, Lutz M, Spek AL, Reek JNH (2005) Zn-II-salphen complexes as versatile building blocks for the construction of supramolecular box assemblies. Chem-Eur J 11:4743–4750. doi:10.1002/chem.200500227
Martínez-Máñez R, Sancenón F (2003) Fluorogenic and chromogenic chemosensors and reagents for anions. Chem Rev 103:4419–4476. doi:10.1021/cr010421e
Luecke H, Quiocho FA (1990) High specificity of a phosphate transport protein determined by hydrogen bonds. Nature 347:402–406. doi:10.1038/347402a0
Djedovic N, Ferdani R, Harder E, Pajewska J, Pajewski R, Weber ME, Schlesinger PH, Gokel GW (2005) The C- and N-terminal residues of synthetic heptapeptide ion channels influence transport efficacy through phospholipid bilayers. New J Chem 29:291–305. doi:10.1039/b417091c
Sivakumar R, Reena V, Ananthi N, Babu M, Anandan S, Velmathi S (2010) Colorimetric and fluorescence sensing of fluoride anions with potential salicylaldimine based schiff base receptors. Spectrochim Acta A Mol Biomol Spectrosc 75:1146–1151. doi:10.1016/j.saa.2009.12.077
Xuan Zh, Lin G, Fang-Ying W, Jiang Y-B (2003) Development of fluorescent sensing of anions under excited-state intermolecular proton transfer signaling mechanism. Org Lett 5:2667–2670. doi:10.1021/ol034846u
Winstanley KJ, Sayer AM, Smith DK (2006) Anion binding by catechols - an NMR, optical and electrochemical study. Org Biomol Chem 4:1760–1767. doi:10.1039/B516433h
Winstanley KJ, Smith DK (2007) Ortho-substituted catechol derivatives: the effect of intramolecular hydrogen-bonding pathways on chloride anion recognition. J Org Chem 72:2803–2815. doi:10.1021/Jo0623989
Hynes JT, Tran-Thi T-H, Granucci G (2002) Intermolecular photochemical proton transfer in solution: new insights and perspectives. J Photochem Photobio A: Chem 154:3–11. doi:10.1016/s1010-6030(02)00304-0
Jarczewski A, Hubbard CD (2003) A review of proton transfer reactions between various carbon-acids and amine bases in aprotic solvents. J Mol Struc 649:287–307. doi:10.1016/s0022-2860(03)00086-3
Arnaut LG, Formosinho SJ (1993) Excited-state proton transfer reactions. I. fundamentals and intermolecular reactions. J Photochem Photobiol A: Chem 75:1–20. doi:org/10.1016/1010-6030(93)80157-5
Abraham Y, Salman H, Suwinska K, Eichen Y (2011) Cyclo 2 benzimidazole: luminescence turn-on sensing of anions. Chem Commun 47:6087–6089. doi:10.1039/c1cc10995b
Xu Y, Pang Y (2010) Zinc binding-induced near-IR emission from excited-state intramolecular proton transfer of a bis(benzoxazole) derivative. Chem Commun 46:4070–4072. doi:10.1039/c003230a
Gong W, Harigae J, Seo J, Lee SS, Hiratani K (2008) Controllable synthesis, structures of amidecrownophane-type macrocycles and their binding ability toward anions. Tetrahedron Lett 49:2268–2271. doi:10.1016/j.tetlet.2008.02.019
Dehkordi MN, Bordbar A-K, Mehrgardi MA, Mirkhani V (2011) Spectrophotometric study on the binding of two water soluble schiff base complexes of Mn (III) with ct-DNA. J Fluoresc 21:1649–1658. doi:10.1007/s10895-011-0854-y
Xu KX, Cheng PF, Zhao J, Wang CJ (2011) Enantioselective fluorescent sensors for amino acid derivatives based on BINOL bearing s-tryptophan unit: synthesis and chiral recognition. J Fluoresc 21:991–1000. doi:10.1007/s10895-009-0585-5
Huang W, Su H, Yao S, Lin H, Cai Z, Lin H (2011) A simple and neutral receptor acting as a sensitive and switch-on fluorescent chemosensor for H2PO −4 . J Fluoresc 21:1697–1702. doi:10.1007/s10895-011-0862-y
Saravanakumar D, Devaraj S, Iyyampillai S, Mohandoss K, Kandaswamy M (2008) Schiff's base phenol-hydrazone derivatives as colorimetric chemosensors for fluoride ions. Tetrahedron Lett 49:127–132. doi:10.1016/j.tetlet.2007.11.006
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76. doi:10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U
Valeur B, Pouget J, Ernsting NP (1992) Tuning of photoinduced energy transfer in a bichromophoric coumarin supermolecule by cation binding. J Phys Chem 96:6545–6549. doi:10.1021/j100195a008
Cohen MD, Schmidt GM (1962) Photochromy and thermochromy of anils. J Phys Chem 66:2442–2446. doi:10.1021/j100818a030
Richey F, Becker RS (1968) Spectroscopy and mechanisms of the photo– and thermal reactions of photochromic Anils. J Chem Phys 49:2092–2100. doi:10.1063/1.1670370
Naeimi H, Moradian M (2010) Synthesis and characterization of nitro-Schiff bases derived from 5-nitro-salicylaldehyde and various diamines and their complexes of Co(II). J Coord Chem 63:156–162. doi:10.1080/00958970903225866
Lopez MV, Bermejo MR, Vazquez ME, Taglietti A, Zaragoza G, Pedrido R, Martinez-Calvo M (2010) Sulfonamide-imines as selective fluorescent chemosensors for the fluoride anion. Org Biomol Chem 8:357–362. doi:10.1039/B916040j
Gomez DE, Fabbrizzi L, Licchelli M, Monzani E (2005) Urea vs. thiourea in anion recognition. Org Biomol Chem 3:1495–1500. doi:10.1039/B500123d
Pfeffer FM, Lim KF, Sedgwick KJ (2007) Indole as a scaffold for anion recognition. Org Biomol Chem 5:1795–1799. doi:10.1039/B702804k
Duke RM, O'Brien JE, McCabe T, Gunnlaugsson T (2008) Colorimetric sensing of anions in aqueous solution using a charge neutral, cleft-like, amidothiourea receptor: tilting the balance between hydrogen bonding and deprotonation in anion recognition. Org Biomol Chem 6:4089–4092. doi:10.1039/b807579d
Sasaki S, Mizuno M, Naemura K, Tobe Y (2000) Synthesis and anion-selective complexation of cyclophane-based cyclic thioureas. J Org Chem 65:275–283. doi:10.1021/jo991237k
Chowdhury P, Panja S, Chakravorti S (2003) Excited state prototropic activities in 2-hydroxy 1-naphthaldehyde. J Phys Chem A 107:83–90. doi:10.1021/jp026404q
Mehata MS, Joshi HC, Tripathi HB (2002) Complexation of 6-hydroxyquinoline with trimethylamine in polar and non-polar solvents. Chem Phys Lett 366:628–635. doi:10.1016/S0009-2614(02)01579-8
Boiocchi M, Boca LD, Gómez DEG, Fabbrizzi L, Licchelli M, Monzani E (2004) Nature of urea-fluoride interaction: incipient and definitive proton transfer. J Am Chem Soc 126:16507–16514. doi:10.1021/ja045936c
Acknowledgement
We are grateful to National Natural Science Foundation of China (No. 21002069) and Doctoral Science Foundation (No. 52LX26).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 533 kb)
Rights and permissions
About this article
Cite this article
Liu, K., Huo, J., Zhu, B. et al. Fluoride-Triggered ESPT in the Binding with Sal(oph)en. J Fluoresc 22, 1231–1236 (2012). https://doi.org/10.1007/s10895-012-1063-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-012-1063-z