Abstract
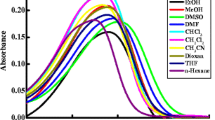
The absorption and fluorescence spectra of 1,5-diaminoanthraquinone(1,5-DAAQ) have been investigated in organic solvents-Benzene(BZ), Ethanol (ETOH), Acetonitrile (AN), Dimethylformamide (DMF) and Dimethyl sulfoxide (DMSO). There is an intra molecular hydrogen bond formed between quinoid oxygen and the substituents NH2 [C = O...H-N]. The interaction of the hydrogen atom of - NH2 leads to red shift in both absorption and fluorescence spectra. The dipole moment ratio of 1,5 DAAQ in ground and excited states was calculated from stokes shift obtained from optical absorption and fluorescence spectra. Photo physical properties of 1,5-DAAQ dye was studied using this absorption and fluorescence spectroscopy techniques in binary liquid mixtures(AN + DMF, AN + DMSO, AN + ETOH and BZ + ETOH).










Similar content being viewed by others
References
Krishnakumar V, John Xavier R (2005) Spectrochimi Acta Part A 61:1799
Siva Kumar P, Kothai Nayaki S, Swaminathan M (2007) E J Chem 4(4):523
Fayed T, Etaiw SEH (1998) Spectrochimi Acta Part A 54:1909
Del Giacco T, Latterini L, Elisei F (2003) J Photochem Photobiol Sci 2:681
Kh PN (2006) High Energy Chem 40(1):22
Kothainayaki S, Swaminathan M (1994) J Photochem Photobiol A: Chem 84:13
Maitra A, Bagchi S (2008) J Mol Liq 137:131
Inamdar SR, Nadaf YF, Mulimani BG (2003) J Mol Struc (Theochem) 624:47
Umadevi M, Ramakrishnan V (2003) J Raman Spectrosc 34:13
Sasirekha V, Vanelle P, Terme T, Ramakrishnan V (2008) J Fluores 9:428
Smith TP, Zaklika KA, Thakur K, Walker GC, Tominaga K, Barbara PF (1991) J Phys Chem 95:10465
Flom SR, Barbara PF (1985) J Phys Chem 89:4489
Acree WE Jr, Tucker SA, Wilkins DC (1993) J Phys Chem 97:11199
Markarian SA, Gabrielian LS, Bonora S (2007) Spectrochim Acta Part A 68:1296
Santo M, Cattana R, Silber JJ (2001) Spectrochim Acta Part A 57:1541
Acknowledgement
One of the authors (VRK) is thankful to CSIR Government of India for the financial assistance provided in the form of a research project. The authors are thankful to Prof. S. Shamuga Sundaram Department of Micro-bio technology, Madurai Kamaraj University for permitting to make use of the Spectrofluorimeter. UGC, Government of India is gratefully acknowledged for providing the Rajeev Gandhi national fellowship to one of the authors (GS). The authors are grateful to UGC for the financial support extended under DRS Phase III for establishing UV-Visible spectrometer facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suganthi, G., Meenakshi, C. & Ramakrishnan, V. Preferential Solvation Studies of 1, 5 Diamino Anthraquinone in Binary Liquid Mixtures. J Fluoresc 20, 95–103 (2010). https://doi.org/10.1007/s10895-009-0527-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-009-0527-2