Abstract
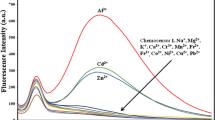
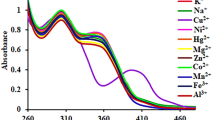
A highly selective PET fluorescent sensor B1 for Hg2+ containing a BODIPY fluorophore and a NS2O2 penta-chelating receptor has been synthesized and characterized. Its absorption maximum wavelength (498 nm) and emission maximum wavelength (512 nm) are both in the visible range. The fluorescence quantum yields of the B1 and Hg2+-bound states of BHg1 are 0.008 and 0.58 in 70% aqueous ethanol solution, respectively. The pKa of 1.97 is the lowest in metal ions PET chemo sensors reported up till now as we know. Thus, B1 can detect the Hg2+ in a wide pH span, which indicates that it has more potential and further practical applications for biology and toxicology. Furthermore, BHg1 also displays response to some anions such as Cl−(Br−), \(CO_3^{2 - } \), SCN− and CH3COO−, which is attributed to the significant coordinating ability of these anions to Hg2+.









Similar content being viewed by others
References
Magos L (1997) Metal ions in biological systems (mercury and its effects on environment and biology). Physiol Toxicol Mercury 34:321–370
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17(2):146–160
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18(3):149–175
Renzoni A, Zino F, Franchi E (1998) Mercury levels along the food chain and risk for exposed populations. Environ Res 77(2):68–72
Mercury Update: Impact on Fish Advisories. EPA Fact Sheet EPA-823-F-01-011; (2001), EPA, Office of Water: Washington, DC
von Burg R, Greenwood MR (1991) In: Merian E (ed) Metals and their compounds in the environment. VCH, Weinheim, pp 1045–1088
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40(12):1335–1351
Harris HH, Pickering I, George GN (2003) Brevia: the chemical form of mercury in fish. Science (Wash. D.C.) 301(5637):1203
Richardson SD, Temes TA (2005) Water analysis: emerging contaminants and current issues. Anal Chem 77(12):3807–3838
Von Burg R (1995) Inorganic mercury-toxicology update. J Appl Toxicol 15(6):483–493
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury-current exposures and clinical manifestations. N Engl J Med 349(18):1731–1737
Welz B, Sperling M (1999) Atomic absorption spectrometry, 3rd ed. Weinheim, Germany, Wiley
Moreton JA, Delves HT (1998) Simple direct method for the determination of total mercury levels in blood and urine and nitric acid digests of fish by inductively coupled plasma mass spectrometry. J Anal At Spectrom 13(7):659–665
Lloris JM, Martinez-Manez R, Padilla-Tosta ME, Pardo T, Soto J, Beer PD, Cadman L, Smith DK (1999) Cyclic and open-chain aza-oxa ferrocene-functionalised derivatives as receptors for the selective electrochemical sensing of toxic heavy metal ions in aqueous environments. J Chem Soc Dalton Trans 14:2359–2370
Jimenez D, Martinez-Manez R, Sancenon F, Soto J (2004) Fluorescent sensor for redox environment: a redox controlled molecular device based on the reversible mercury mediated folded structure formation of oligothymidylate. Tetrahedron Lett 45(6):1257–1259
Youn NJ, Chang S-K (2005) Dimethylcyclam based fluoroionophore having Hg2 +- and Cd2 +-selective signaling behaviors. Tetrahedron Lett 46(1):125–129
Moon S-Y, Youn NJ, Park SM, Chang S-K (2005) Diametrically disubstituted cyclam derivative having Hg2+-selective fluoroionophoric behaviors. J Org Chem 70(6):2394–2397
Miyake Y, Ono A (2005) Fluorescent sensor for redox environment: a redox controlled molecular device based on the reversible mercury mediated folded structure formation of oligothymidylate. Tetrahedron Lett 46(14):2441–2443
Sancenon F, Martinez-Manez R, Soto J (2001) 1,3,5-Triarylpent-2-en-1,5-diones for the colorimetric sensing of the mercuric cation. Chem Commun 21:2262–2263
Choi MJ, Kim MY, Chang S-K A new Hg2+-selective chromoionophore based on calix[4]arenediazacrown ether. Chem Commun 17:1664–1665
Guo X, Qian X, Jia L (2004) A highly selective and sensitive fluorescent chemosensor for Hg2+ in neutral buffer aqueous solution. J Am Chem Soc 126(8):2272–2273
Yoon S, Albers AE, Wong AP, Chang CJ (2005) Screening mercury levels in fish with a selective fluorescent chemosensor. J Am Chem Soc 127(46):16030–16031
Zheng H, Qian Z-H, Xu L, Yuan F-F, Lan L-D, Xu J-G (2006) Switching the recognition preference of Rhodamine B Spirolactam by replacing one atom: design of Rhodamine B Thiohydrazide for recognition of Hg(II) in aqueous solution. Org Lett 8(5):859–861
Ko S-K, Yang Y-K, Tae J, Shin I (2006) In vivo monitoring of mercury ions using a Rhodamine-based molecular probe. J Am Chem Soc 128(43):14150–14155
Nolan EM, Lippard SJ (2003) A “Turn-On” fluorescent sensor for the selective detection of mercuric ion in aqueous media. J Am Chem Soc 125(47):14270–14271
Nolan EM, Racine ME, Lippard SJ (2006) Selective Hg(II) detection in aqueous solution with Thiol derivatized fluoresceins. Inorg Chem 45(6):2742–2749
Nolan EM, Lippard SJ (2007) Turn-on and ratiometric mercury sensing in water with a red-emitting probe. J Am Chem Soc 129(18):5910–5918
Nolan EM, Jaworski J, Okamoto K-I, Hayashi Y, Sheng M, Lippard SJ (2005) QZ1 and QZ2: rapid, reversible quinoline-derivatized fluoresceins for sensing biological Zn(II). J Am Chem Soc 127(48):16812–16823
Goldsmith CR, Lippard SJ (2006) Analogues of Zinpyr-1 provide insight into the mechanism of zinc sensing. Inorg Chem 45(16):6474–6478
Jin Y, Yoon I, Seo J, Lee J-E, Moon S-T, Kim J, Han SW, Park K-M, Lindoy LF, Lee SS (2005) Cadmium(II) and mercury(II) complexes of an NO2S2-donor macrocycle and its ditopic xylyl-bridged analogue. Dalton Trans 4:788–796
Lee SJ, Jung JH, Seo J, Yoon I, Park K-M, Lindoy LF, Lee SS (2006) A chromogenic macrocycle exhibiting Cation-selective and anion-controlled color change: an approach to understanding structure-color relationships. Org Lett 8(8):1641–1643
Yoon S, Albers AE, Wong AP, Chang CJ (2005) Screening mercury levels in fish with a selective fluorescent chemosensor. J Am Chem Soc 127(46):16030–16031
Masuhara H, Shioyama H, Saito T, Hamada K, Yasoshima S, Mataga N (1984) Fluorescence quenching mechanism of aromatic hydrocarbons by closed-shell heavy metal ions in aqueous and organic solutions. J Phys Chem 88(24):5868–5873
Rurack K, Kollmannsberger M, Resch-Genger U, Daub J (2000) A selective and sensitive fluoroionophore for HgII, AgI, and CuII with virtually decoupled fluorophore and receptor units. J Am Chem Soc 122(5):968–969
Karolin J, Johansson LB-A, Strandberg L, Ny T (1994) J Am Chem Soc 116(17):7801–7806
Cui A, Peng X, Fan J, Chen X, Wu Y, Guo B (2007) Synthesis, spectral properties and photostability of novel boron-dipyrromethene dyes. J Photochem Photobiol A Chem 186(1):85–92
Guo B, Peng X, Cui A, Wu Y, Tian M, Zhang L, Chen X, Gao Y (2007) Synthesis and spectral properties of new boron dipyrromethene dyes. Dyes Pigm 73(2):206–210
Peng X, Du J, Fan J, Wang J, Wu Y, Zhao J, Sun S, Xu T (2007) A selective fluorescent sensor for imaging Cd2+ in living cells. J Am Chem Soc 129(6):1500–1501
Wu Y, Peng X, Guo B, Fan J, Zha, ng Z, Wang J, Cui A, Gao Y (2005) Boron dipyrromethene fluorophore based fluorescence sensor for the selective imaging of Zn(II) in living cells. Org Biomol Chem 3(8):1387–1392
Baruah M, Qin W, Basaric N, De Borggaeve WM, Boens N (2005) BODIPY-based hydroxyaryl derivatives as fluorescent ph probes. J Org Chem 70(10):4152–4157
Acknowledgements
This work was supported by the National Science Foundation of China (20376010 and 20472012,).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Du, J., Fan, J., Peng, X. et al. Highly Selective and Anions Controlled Fluorescent Sensor for Hg2+ in Aqueous Environment. J Fluoresc 18, 919–924 (2008). https://doi.org/10.1007/s10895-008-0324-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-008-0324-3