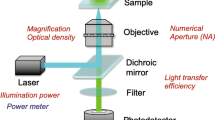
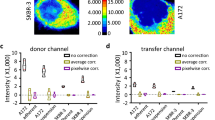
Abstract
The scope of this paper is to illustrate the need for an improved quality assurance in fluorometry. For this purpose, instrumental sources of error and their influences on the reliability and comparability of fluorescence data are highlighted for frequently used photoluminescence techniques ranging from conventional macro- and microfluorometry over fluorescence microscopy and flow cytometry to microarray technology as well as in vivo fluorescence imaging. Particularly, the need for and requirements on fluorescence standards for the characterization and performance validation of fluorescence instruments, to enhance the comparability of fluorescence data, and to enable quantitative fluorescence analysis are discussed. Special emphasis is dedicated to spectral fluorescence standards and fluorescence intensity standards.
Similar content being viewed by others
References
J. R. Lakowicz (Ed.) (1999). Principles of Fluorescence Spectroscopy, 2nd edn., Kluwer Academic/Plenum Press, New York.
J. R. Lakowicz (Ed.) (1992–2004). Topics in Fluorescence Spectroscopy Series, Vols. 1–8, Plenum Press, New York.
O. S. Wolfbeis (Series Ed.) (2001–2004). Springer Series on Fluorescence, Methods and Applications, Vols. 1–3, Springer, Berlin.
S. G. Schulman (Ed.) (1985–1993). Molecular Luminescence Spectroscopy Parts, Vols. 1–3, Wiley Interscience, New York.
W. T. Mason (1999). Fluorescent and Luminescent Probes for Biological Activity, 2nd edn., Academic Press, San Diego.
A. J. Pope, U. M. Haupts, and K. J. Moore (1999). Homogenous fluorescence readouts for miniaturized high-throughput screening: Theory and practice. Drug Discov. Today 4(8), 350–362.
E. Zubritsky (1999). Microplate readers reach critical mass. Anal. Chem. News Features 71, 39A–43A.
C. T. Wittwer, K. M. Ririe, R. V. Andrew, D. A. David, R. A. Gundry, and U. J. Balis (1997). The light cycler: A microvolume multisample fluorimeter with rapid temperature control. Biotechniques 22, 176–181.
Supplement (1998). Fluoreszenzspektroskopie. Nachr. Chem. Tech. Lab. 46, S121–S132.
Analytical Methods Committee (1998). Evaluation of analytical instrumentation. Part XI Instrumentation for molecular fluorescence spectrometry. Analyst 123, 1649–1656.
J. W. Eastman (1966). Standardization of fluorescence spectra and the calibration of spectrofluorimeters. Appl. Optics 5(7), 1125–1132.
W. Galbraith, K. W. Ryan, N. Gliksman, D. L. Taylor, and A. S. Waggoner (1989). Multiple spectral parameter imaging in quantitative fluorescence microscopy. I: Quantitation of bead standards. Comput. Med. Imag. Graph. 13, 47–60.
A. K. Gaigalas, L. Li, O. Henderson, R. Vogt, J. Barr, G. Marti, J. Weaver, and A. Schwartz (2001). The development of fluorescence intensity standards. J. Res. Natl. Inst. Stand. Technol. 106(2), 381–389.
C. A. Parker (1968). Photoluminescence of Solutions, Elsevier, Amsterdam.
J. N. Miller (1981). Standards in Fluorescence Spectrometry, Ultraviolett Spectrometry Group, London.
D. F. Eaton (1988). Reference materials for fluorescence measurement. Pure Appl. Chem. 60, 1107–1114.
R. A. Velapoldi and M. S. Epstein (1989). In M. C. Goldberg (Ed.), Luminescence Applications in Biological, Chemical, Environmental and Hydrological Sciences, ACS Symposium Series, Vol. 383, American Chemical Society, Washington, DC, pp. 98– 126.
W. D. Niles and F. S. Cohen (1995). Radiometric calibration of a video fluorescence microscope for the quantitative imaging of resonance energy transfer. Rev. Sci. Instrum. 66, 3527– 3536.
EN ISO/IEC 17025; GLP/GMP, GLP: Good laboratory praxis; GMP: Good manufacturing praxis.
Burgess and D. G. Jones (1995). Spectrophotometry, Luminescence and Colour: Science and Compliance, Elsevier, Amsterdam.
O. D. D. Soares and J. L. C. Costa (1999). Spectrophotometers intercomparison for spectrocolorimetric scale harmonization. Rev. Sci. Instrum. 70(12), 4471–4481.
J. C. Travis, J. C. Zwinkels, F. Mercader, A. Ruiz, E. A. Early, M. V. Smith, M. Noel, M. Maley, G. W. Kramer, K. L. Eckerle, and D. L. Duewer (2002). An international evaluation of holmium oxide solution reference materials for wavelength calibration in molecular absorption spectrophotometry. Anal. Chem. 74, 3408–3415.
ASTM E 169-87 (reapproved 2003). Standard Practices for general Techniques of Ultraviolet-Visible Quantitative Analysis, and herein referenced ASTM standards.
CIE-Publ. 15.2, Colorimetry, 2nd edn., 1986.
K. D. Mielenz (1987). In C. Burgess and K. D. Mielenz (Eds.), Advances in Standards and Methodology in Spectrophotometry, Elsevier, Amsterdam, pp. 49–62.
D. C. Rich and D. Martin (1999). Improved model for improving the inter-instrument agreement of spectrocolorimeters. Anal. Chim. Acta 380, 263–276.
K. Witt (2002/2003). Haben wir die UV/vis-Spektrometerie lumineszierender Materialien im Griff? Die Farbe 46(1/2), 31–51.
ASTM E 388-72 (reapproved 2003). Spectral bandwidth and wavelength accuracy of fluorescence spectrometers.
ASTM E 578-83 (reapproved 2003). Linearity of fluorescence measuring system.
ASTM E 579-84 (reapproved 2003). Limit of detection of fluorescence of quinine sulfate.
A. Schwartz and E. Fernandez-Repollet (1993). Development of clinical standards for flow cytometry. Ann. N. Y. Acad. Sci. 677, 28–39.
A. Schwartz, M. Mendez, G. Santiago, L. Diaz, and E. Fernandez-Repollet (1997). Applications of common quantitative fluorescent standards to multiple platforms: Comparison of commercial fluorescent calibration standards used in quantitative flow cytometry. Clin. Immunol. 17(1), 14–18.
A. Schwartz, G. E. Marti, J. W. Gratama, and E. Fernandez-Repollet (1998). Standardizing flow cytometry: A classification system of fluorescence standards used for flow cytometry. Cytometry 33, 106–114.
J. W. Gratama, J. L. D’Hautcourt, F. Mandy, G. Rothe, D. Barnett, G. Janossy, S. Papa, G. Schmitz, and R. Lenkei (1998). Flow cytometric quantitation of immunofluorescence intensity: Problems and perspectives. Cytometry 33, 166–178.
R. A. Velapoldi (1987). Liquid standards in fluorescence spectrometry. In C. Burgess and K. D. Mielenz (Eds.), Advances in Standards and Methodology in Spectrophotometry, Elsevier, Amsterdam, pp. 175–193.
I. Billard, E. Ansoborlo, K. Afferson, S. Arpigny, M. E. Azenha, D. Brich, P. Bros, H. D. Burrows, G. Choppin, L. Couston, V. Dubois, T. Fangh¨nel, G. Geipel, S. Hubert, J. I. Kim, T. Kimura, R. Klenze, A. Kronenberg, M. Kumke, G. Lagarde, G. Llamarque, S. Lis, C. Madic, G. Meinrath, C. Moulin, R. Nagaishi, D. Parker, G. Plancque, F. Scherbaum, E. Simoni, S. Sinkov, and C. Viallesoubranne (2003). Appl. Spectrsc. 57(8), 1027–1038.
For clear definition of the metrological hierarchy of reference materials, see International Vocabulary of Basics and General Terms in Metrology (1994), 2nd edn., Beuth Verlag GmbH.
R. A. Velapoldi and K. D. Mielenz (1980). A fluorescence standard reference material: Quinine sulfate dihydrate, NBS Spec. Publ. 260–264, PB 80132046, Springfield, VA.
A. Schwartz, L. Wang, E. Early, A. Gaigalas, Y.-Z. Zhang, G. E. Marti, and R. F. Vogt (2002). Quantitating fluorescence intensity from fluorophore: The definition of MESF assignment. J. Res. Natl. Inst. Stand. Technol. 107(1), 83–91.
U. Resch-Genger, D. Pfeifer, C. Monte, W. Pilz, A. Hoffmann, M. Spieles, J. Hollandt, R. D. Taubert, B. Sch¨nenberger, P. Nording (in press), Traceability of fluorometry. Part II: Spectral fluorescence standards. J. Fluoresc. 15(3), 325.
J. W. Verhoeven (1996). Molecular terms used in photochemistry (recommendations 1996). Pure Appl. Chem. 68(12), 2223–2286.
J. Hollandt, R. D. Taubert, J. Seidel, A. Gugg-Helminger, U. Resch-Genger, D. Pfeifer, C. Monte, and W. Pilz (in press), Traceability of fluorometry. Part I: Physical standards. J. Fluoresc. 15(3), 311.
E. D. Cehelnik, K. D. Mielenz, and R. A. Velapoldi (1975). Polarization effects on fluorescence measurements. J. Res. Natl. Bur. Stand. A 79A(1), 1–15.
U. Resch-Genger, K. Hoffmann, and A. Engel, manuscript in preparation.
T. Erdogan, A. Pradhan, and V. Mizrahi (2003). Optical filters impact fluorescence fidelity. Biophotonics Int. 10(10), 38–43.
J.W. Hofstraat and M. J. Latuhihin (1994). Correction of fluorescence spectra. Appl. Spectrosc. 48(4), 436–446.
J. A. Gardecki and M. Maroncelli (1998). Set of secondary emission standards for calibration of the spectral responsivity in emission spectroscopy. Appl. Spectrosc. 52(9), 1179–1189.
R. J. Kovach and W. M. Peterson (1994). The measurement of sensitivity in fluorescence spectroscopy. Am. Lab. 32G–32K.
R. B. Thompson, I. Gryczynski, and J. Malicka (2002). Fluorescence polarization standards for high-throughput screening and imaging. Biotechniques 32(1), 34–41.
See for instance Molecular Probes, Starna GmbH, Matech Precision Dynamics Coorp., Labsphere Inc., Fluka GmbH, LambdaChem GmbH, and SUMITA Optical Glass Inc. as well as NIST (RM 8640, SRM 936a, and 1932).
R. F. Chen (1972). Measurement of absolute values in biochemical fluorescence spectroscopy. J. Res. Nat. Bur. Stand. A 76(6), 593–606.
R. B. Thompson, I. Gryczynski, and J. Malicka (2002). Fluorescence polarization standards for high-throughput screening and imaging. Biotechniques 32(1), 34–41.
I. T. Lifshotz and M. L. Meilman (1989). Standard sample for calibrating wavelength scales of spectral fluorimeters. Sov. J. Opt. Technol. 55(8), 487–489.
S. A. Wise, L. C. Sander, and W. E. May (1993). Determination of polycyclic aromatic hydrocarbons by liquid chromatography. J. Chromatogr. 642, 329–349.
SRM 1647b, NIST.
A. Schwartz, A. K. Gaigalas, L. Wang, G. E. Marti, R. F. Vogt, and E. Fernandez-Repollet (2004). Formalization of the MESF unit of fluorescence intensity. Cytometry 57B(1), 1–6.
RM 8640, NIST.
P. Froehlich (1989). Under the sensitivity specification for a fluorescence spectrophotometer. Int. Lab. 42–44.
R. J. Kovach and W. M. Peterson (1994). The measurement of mensitivity in fluorescence spectroscopy. Am. Lab. 32G–32K.
ISO (1993). Guide to the expression of uncertainty in measurement.
J. N. Demas (1982). In K. D. Mielenz (Ed.), Optical Radiation Measurements, Vol. 3, Academic Press, New York, p. 195.
W. Geffken (1962). The molar absorption of different ions in glasses. Glastechn. Berichte 35, 27–35.
M. Mizuguchi, H. Hosono, and H. Kawazone (1999). Time-resolved photoluminescence for diagnostic of photoluminescence to ArF excimer laser damage to CaF2 single crystals. J. Opt. Soc. Am. 7(16), 1153–1159.
P. de Rose (2003). Bioanalytical and biomedical applications of fluorescence techniques: Instrument characterization and validation, traceability, and need for reference materials, in Fluorescence Workshop, BERM-9, Berlin.
D. Ehrt, P. Ebeling, U. Natura, U. Kohlberg, K. Naumann, and S. Ritter (2001). Redox equilibria and ultraviolet radiation induced defects in glasses. Int. Cong. Glass 1, 84–96.
J. W. Chan, T. Huster, J. S. Hayden, S. H. Risbud, and D. M. Krol (2002). J. Am. Ceram. Soc. 85(5), 1037–1040.
A. Engel, K. Knapp, B. Speit, G. Wehrhan, and E. M¨rsen (2001). High quality CaF2 used for 157 nm micro lithography. Fab. Tech. 14, 177–184.
C. M¨hlig, W. Triebel, G. T¨pfer, and A. Jordanov (2003). CaF2 for ArF lithography—Characterisation by in-situ and LIF measurements, CHOCLAB II final report—Optics Characterization, pp. 257–267.
A. Engel, W. Triebel, C. M¨hlig, J. Alkemper, A. Kr¨mer, J. Kandler, K. Knapp, and E. M¨rsen (2000). Visualization of laser damage in 157 nm material CaF2 and BaF2, 1st 157 nm Symposium, Dana Point CA, pp. 391–398.
A. Engel, R. Haspel, and V. Rupertus (2003). Advanced industrial fluorescence metrology used for qualification of high-quality optical materials. SPIE Proc. 5118–5120, 182–189.
R. A. Velapoldi (1971). Fluorescence. Nat. Bur. Stand. Tech. Note 584, 53–83.
H. Pick (1972). Structure of trapped electron and trapped hole centers in alkali halides. In J. Abeles (Ed.), Optical Properties of Solids, North Holland, pp. 653–668.
D. W. Pack, W. J. Manthey, and D. S. Mc Clure (1989). Production of color centers with ionizing irradiation in alkali halides. Phys. Rev. B 40(14), 9930–9935.
M. Letz, A. Engel, L. Parthier, U. Natura, and K. Knapp (2004). CaF2 for DUV lens fabrication: Basic material properties and dynamic light-matter interaction. SPIE Proc. 5377, Optical Microlithography XVII, 1797–1804.
Japanese Optical Glass Industrial Standards, JOGIS, 03-1975.
W. Gellermann (1989). J. Chem. Solids 52, 249–254.
W. Goehde, U. Cassens, L. G. Lehman, Y. Traore, W. Goehde jun., P. Berkes, C. Westerberg, and B. Greve (2003). Individual patient-dependent influence of erythrocyte lysing procedures on flow-cytometric analysis of leukocyte subpopulations. Transfusion Med. Hemother. 30, 165–170.
J.-C. Strohmeyer, C. Blume, C. Meisel, W.-D. Doecke, M. Hummel, C. Hoeflich, K. Thiele, A. Unbehaun, R. Hetzer, and H.-D. Volk (2003). Standardized immune monitoring for the prediction on infections after cardiopulmonary bypass surgery in risk patients. Cytometry 53B, 54–62.
G. Monneret, N. Elmenkouri, J. Bohe, A. L. Debard, A. C. Gutowski, J. Bienvenu, and A. Lepape (2002). Analytical requirements for measuring monocytic human lymphocyte antigen DR by flow cytometry: Application to the monitoring of patients with septic shock. Clin. Chem. 48, 1589–1592.
S. B. Iyer, M. J. E. Bishop, B. Abrams, V. C. Maino, A. J. Ward, T. P. Christion, and K. A. Davis, QuantiBRITE™: A new standard for fluorescence quantification, http://www.bdbiosciences.com/immunocytometry_systems (see download literature, White Papers, QuantiBRITE™ White Paper).
Y. Gerena-López, J. Nolan, L. Wang, A. Gaigalas, A. Schwartz, and E. Fernández-Repollet (2004). Quantification of EGFP expression on Molt-4 T cells using calibration standards. Cytometry 60A, 21–28.
J. B. Pawley (Ed.) (1995). Handbook of Biological Confocal Microscopy, 2nd edn., Kluwer Academic Publishers, New York.
S. Inoue (Ed.) (1986). Video Microscopy, Plenum Publishers, New York.
D. B. Murphy (Ed.) (2001). Fundamentals of Light Microscopy and Electronic Imaging, Wiley-Liss, New York.
X. F. Wang, A. Periasamy, B. Herman, and D. M. Coleman (1992). Fluorescence lifetime imaging microscopy (FLIM): Instrumentation and applications. Crit. Rev. Anal. Chem. 23(5), 369– 395.
Special issue on the use of ion-sensitive fluorophores for making accurate intracellular ion measurements at high spatial and/or temporal resolution (1990). Cell Calcium, February/March.
M. Andreeff and D. Pinkel (Ed.) (1999). Introduction to Fluorescence in Situ Hybridization: Principals and Clinical Applications, Wiley-Liss, New York.
V. E. Centonze, A. Takahashi, E. Casanova, and B. Herman (2000). Quantitative fluorescence microscopy. J. Histotechnol. 23(3), 229–234.
W. D. Niles and F. C. Cohen (1995). Radiometric calibration of a video fluorescence microscope for quantitative imaging of resonance energy transfer. Rev. Sci. Instrum. 66(6), 3527–3536.
R. Nitschke (2004). Standardization and quantification in microscopy, Workshop AK PhotonicNet, Wetzlar.
The point-spread function is determined by the product of the excitation intensity distribution and the light collection efficiency function.
J. S. Ploem (1970). Standards for fluorescence microscopy, in E. J. Holborow (Ed.), Standard for Immunofluorescence Symposium, Blackwell Scientific Publications, Oxford, pp. 137–153.
W. Galbraith, K. W. Ryan, N. Gliksman, D. Lansing Taylor, and A. S. Waggoner (1989). Multiple spectral parameter imaging in quantitative fluorescence microscopy. I: Quantitation of bead standards. Comp. Med. Imag. Graphics 13(1), 47–60.
J. M. Lerner and R. M. Zucker (2004). Calibration and validation of confocal spectral imaging systems. Cytometry 62A, 8–34.
M. Sernetz and A. Thaer (1970). A capillary fluorescence standard for microfluorometry. J. Microscopy 91(1), 43–52.
F. W. D. Rost (1991). Quantitative Fluorescence Microscopy, Cambridge University Press, Cambridge, p. 236.
D. S. Kaplan and G. L. Picciolo (1989). Characterization of instrumentation and calibrators for quantitative microfluorometry for immunofluorescence tests. J. Clin. Microbiol. 27, 442–447.
A. P. M. Jongsma, W. Hijmans, and J. S. Ploem (1971). Quantitative immunofluorescence. Histochemie 25, 329–343.
R. A. Velapoldi, J. C. Travis, W. A. Cassatt, and W. T. Yap (1975). Inorganic ion-doped glass fibres as microspectrofluorimetric standards. J. Microsc. 103(3), 293–303.
R. P. Haugland (Ed.) (2002). Handbook of Fluorescent Probes and Research Products, 9th edn., Molecular Probes, Section 24.1.
J. J. Haaijman and J. P. R. van Dalen (1974). Quantification in immunofluorescence microscopy: A new standard for fluorescein and rhodamine emission measurement. J. Immunol. Methods 5, 359–374.
S. J. Lockett, K. Jacobson, and B. Herman (1992). Quantitative precision of an automated fluorescence-based image cytometer. Anal. Quant. Cytol. Histol. 14, 187–202.
M. A. Model and J. K. Burkhardt (2001). A standard for calibration and shading correction of a fluorescence microscope. Cytometry 44, 309–316.
J. E. Sisken (1989). Fluorescent standards. Methods Cell Biol. 30, 113–126.
S. G. Turney, S. M. Culican, and J. W. Lichtman (1996). A quantitative fluorescence-imaging technique for studying acetylcholine receptor turnover at neuromuscular junctions in living animals. J. Neurosci. Methods 64, 199–208.
P. W. Stevens and D. M. Kelso (2003). Imaging and analysis of immobilized particle arrays. Anal. Chem. 75, 1147–1154.
A. C. Jones, M. Millington, J. Muhl, J. M. De Freitas, J. S. Barton, and G. Gregory (2001). Calibration of an optical fluorescence method for film thickness measurement. Meas. Sci. Technol. 12, N23–N27.
Evident Technologies, Product Catalogue, February 2004.
A. P. Alivisatos (1996). Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 934–937.
A. Knight, J. Gaunt, T. Davidson, V. Chechik, and S. Windsor (2004). Evaluation of the suitability of quantum dots as fluorescence standards. NPL report DQL-AS 007.
W. G. J. H. M. van Sark, P. L. T. M. Frederix, D. J. van den Heuvel, H. C. Gerritsen, A. A. Bol, J. N. J. van Lingen, C. de Mello Donega, and A. Meijerink (2001). Photoxidation and photobleaching of single CdSe/ZnS quantum dots probed by room-temperature time resolved spectroscopy. J. Phys. Chem. B 105(10), 8281– 8284.
I. T. Young (1983). The use of digital image processing techniques for the calibration of quantitative microscopes. Proc. SPIE 387, 326–335.
APE GmbH, Berlin; http://www.ape-berlin.de.
M. C. Pirrung (2002). How to make a DNA chip. Angew. Chem. Int. Ed. 41(8), 1276–1289.
J. R. Epstein, I. Biran, and D. R. Walt (2002). Fluorescence-based nucleic acid detection and microarrays. Anal. Chim. Acta 469, 3–36.
B. Lemieux, A. Asharoni, and M. Schena (1998). Overview of DNA chip technology. Mol. Breed. 4, 277–289.
P. Hedge, R. Qi, K. Abernathy, C. Gay, S. Dharap, R. Gaspard, J. E. Hughes, E. Snesrud, N. Lee, and J. Quackenbush (2000). A concise guide to cDNA microarray analysis. Biotechniques 29(3), 548–562.
C. B. V. Christensen (2002). Arrays in biological and chemical analysis. Talanta 56, 289–299.
Biochips (Market survey) (2003). New Drugs 26–29.
M. Schena, D. Shalon, R. W. Davis, and P. O. Brown (1995). Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270(5235), 467–470.
M. B. Eisen, P. T. Spellman, P. O. Brown, and D. Botstein (1998). Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U.S.A. 95(25), 14863–14868.
S. Granjeaud, F. Bertucci, and B. R. Jordan (1999). Expression profiling: DNA arrays in many guises. Bioassays 21(9), 781–790.
M. B. Eisen and P. O. Brown (1999). DNA arrays for analysis of gene expression. Meth. Enzymol. 303, 179–205.
A. Butte (2002). The use and analysis of microarray data. Nat. Rev. Drug Discov. 1, 951–960
M. Taniguchi, K. Miura, H. Iwao, and S. Yamanaka (2001). Quantitative assessment of DNA microarrays—Comparison with Northern Blot analyses. Genomics 71, 34–39.
M. Bartosiewicz, M. Trounstine, D. Barker, R. Jonston, and A. Buckpitt (2000). Development of a toxicological gene array and quantitative assessment of this technology. Arch. Biochem. Biophys. 376(1), 66–73.
E. A. Winzeler, M. Schena, and R. W. Davis (1999). Fluorescence-based expression monitoring using microarrays. Meth. Enzymol. 306, 3–18.
Z. Guo, R. A. Guilfoyle, A. J. Thiel, R. Wang, and L. M. Smith (1994). Direct fluorescence analysis of genetic polymorphism by hybridization with oligonucleotide arrays an glass supports. Nucleic Acids Res. 22(24), 5456–5465.
J. B. Randolph and A. S. Waggoner (1997). Stability, specifity and fluorescence brightness of multiply-labeled fluorescent DNA probes. Nucleic Acids Res. 25(14), 2923–2929.
F. Perraut, A. Lagrange, P. Pouteau, O. Peyssonneaux, P. Puget, G. McGall, L. Menou, R. Gonzalez, P. Labeye, and F. Ginot (2002). A new generation of scanners for DNA chips. Biosens. Bioelectron. 17, 803–813.
K. Adelheim, E. Emantraut, T. Kaiser, and J. Tuchscheerer (2002). Smart chip for array experiment standardization. New Drugs 22–23.
A. M. Dudley, J. Aach, M. A. Steffen, and G. M. Church (2002). Measuring absolute expression with microarrays with a calibrated reference sample and an extended signal intensity range. Proc. Natl. Acad. Sci. U.S.A. 99(11), 7554–7559.
M. R. Weil, T. Macatee, and H. R. Garner (2002). Toward a universal standard: Comparing two methods for standardizing spotted microarray data. Biotechniques 32(6), 1310–1314.
M. Cronin, K. Ghosh, F. Sistare, J. Quackenbush, V. Vilker, and C. O’Connell (2004). Universal RNA Reference Materials for Gene Expression. Clin. Chem. [meeting review].
Y. F. Leung and D. Cavalieri (2003). Fundamentals of cDNA microarray data analysis. Trends Genet. 19(11), 649–659.
T. Forster, D. Roy, and P. Ghazal (2003). Experiments using microarray technology: Limitations and standard operating procedures. J. Endocrinol. 178(2), 195–204.
Tumor Analysis Best Practices Working Group (2004). Expression profiling—Best practices for data generation and interpretation in clinical trials. Nat. Rev. Genet. 5(3), 229–237.
A. T. Weeraratna, J. E. Nagel, V. d. V. Mello-Coelho, and D. D. Taub (2004). Gene expression profiling: from microarrays to medicine. J. Clin. Immunol. 24(3), 213–224.
A. Brazma, P. Hingamp, J. Quackenbush, G. Sherlock, P. Spellman, C. Stoeckert, J. Aach, W. Ansorge, C. A. Ball, H. C. Causton, T. Gaasterland, P. Glenisson, F. C. Holstege, I. F. Kim, V. Markowitz, J. C. Matese, H. Parkinson, A. Robinson, U. Sarkans, S. Schulze-Kremer, J. Stewart, R. Taylor, and J. Vilo (2001). Vingron M. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat. Genet. 29(4), 365–371.
P. T. Spellman, M. Miller, J. Stewart, C. Troup, U. Sarkans, S. Chervitz, D. Bernhart, G. Sherlock, C. Ball, M. Lepage, M. Swiatek, W. L. Marks, J. Goncalves, S. Markel, D. Iordan, M. Shojatalab, A. Pizarro, J. White, R. Hubley, E. Deutsch, M. Senger, B. J. Aronow, A. Robinson, D. Bassett, C. J. Jr. Stoeckert, and A. Brazma (2002). Design and implementation of microarray gene expression markup language (MAGE-ML). Genome Biol. 3(9), RESEARCH0046.
A. Brazma, H. Parkinson, U. Sarkans, M. Shojatalab, J. Vilo, N. Abeygunawardena, E. Holloway, M. Kapushesky, P. Kemmeren, G. G. Lara, A. Oezcimen, P. Rocca-Serra, and S. A. Sansone (2003). ArrayExpress—A public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 31(1), 68–71.
J. Gollub, C. A. Ball, G. Binkley, J. Demeter, D. B. Finkelstein, J. M. Hebert, T. Hernandez-Boussard, H. Jin, M. Kaloper, J. C. Matese, M. Schroeder, P. O. Brown, D. Botstein, and G. Sherlock (2003). The Stanford Microarray Database: Data access and quality assessment tools. Nucleic Acids Res. 31(1), 94–96.
See for example, Clondiag Chip Technologies GmbH; Full Moon Biosystems Inc.
G. A. Wagnières, W. M. Star, and B. C. Wilson (1998). In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem. Photobiol. 68, 603–632.
R. Richards-Kortum and E. M. Sevick-Muraca (1996). Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 47, 555–606.
D. J. Cruccia, F. Bevilacqua, A. J. Durkin, S. Merritt, B. J. Tromberg, G. Gulsen, H. Yu, J. Wang, and O. Nalcioglu (2003). In vivo quantification of optical contrast agent dynamics in rat tumors by use of diffuse optical spectroscopy with magnetic resonance imaging coregistration. Appl. Opt. 42, 2940–2950.
D. Y. Paithankar, A. U. Chen, B. W. Pogue, M. S. Patterson, and E. M. Sevick-Muraca (1997). Imaging of fluorescent yield and lifetime from multiply scattered light reemitted from random media. Appl. Opt. 36, 2260–2272.
R. Weissleder and U. Mahmood (2001). Mol. Imag. Radiol. 219, 316–333.
V. Ntziachristos, J. Ripoll, and R. Weissleder (2002). Would near-infrared fluorescence signals propagate through large human organs for clinical studies? Opt. Lett. 27, 333–335.
R. Weissleder and V. Ntziachristos (2003). Shedding light onto live molecular targets. Nat. Med. 9, 123–128.
M. Rudin and R. Weissleder (2003). Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2, 123– 131.
V. Ntziachhristos, C. Bremer, and R. Weissleder (2003). Fluorescence imaging with near-infrared light: New technological advances that enable in vivo molecular imaging. Eur. Radiol. 13, 195–208.
A. Becker, C. Hessenius, K. Licha, B. Ebert, U. Sukowski, W. Semmler, B. Wiedenmann, and C. Gr¨tzinger (2001). Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat. Biotechnol. 19, 327–331.
E. E. Graves, J. P. Culver, J. Ripoll, R. Weissleder, and V. Ntziachristos (2004). Singular-value analysis and optimization of experimental parameters in fluorescence molecular tomography. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 21, 231–241.
U. Sukowski, F. Schubert, D. Grosenick, and H. Rinneberg (1996). Preparation of solid phantoms with defined scattering and absorption properties for optical tomography. Phys. Med. Biol. 41, 1823–1844.
K. Licha (2002). Contrast agents for optical imaging. Topics Curr. Chem. 222, 22–29.
M.-A. E. J. Ortner, B. Ebert, E. Hein, K. Zumbusch, D. Nolte, U. Sukowski, J. Weber-Eibel, B. Fleige, M. Dietel, M. Stolte, G. Oberhuber, R. Porschen, B. Klump, H. H¨rtnagel, H. Lochs, and H. Rinneberg (2003). Time gated fluorescence spectroscopy in Barrett’s oesophagus. Gut 52, 28–33.
K. T. Moesta, B. Ebert, T. Handke, D. Nolte, C. Nowak, W. E. Haensch, R. K. Pandey, T. J. Dougherty, H. Rinneberg, and P. M. Schlag (2001). Protoporphyrin IX occurs naturally in colorectal cancers and their metastases. Cancer Res. 61, 991– 999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Resch-Genger, U., Hoffmann, K., Nietfeld, W. et al. How to Improve Quality Assurance in Fluorometry: Fluorescence-Inherent Sources of Error and Suited Fluorescence Standards. J Fluoresc 15, 337–362 (2005). https://doi.org/10.1007/s10895-005-2630-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10895-005-2630-3