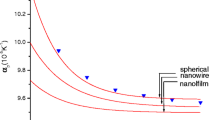
The joint influence of size effects connected with physicochemical processes both on the surface of nanoobjects and in their volume on the phase transformations taking place in them has been investigated theoretically. The joint manifestation of the surface and volume size effects has been analyzed using as an example the growth of silicon nanowhiskers.
Similar content being viewed by others
References
F. Dhalluin, P. J. Destre, M. I. den Hertog, J.-L. Rouviere, P. Ferret, P. Gentile, and T. Baron, Critical condition for growth of silicon nanowires, J. Appl. Phys., 102, 094906-1–094906-5 (2007).
A. G. Nastov’yak, I. G. Neizvestnyi, N. L. Shvarts, and Z. Sh. Yanovitskaya, Simulation of the growth of nanowhiskers by the Monte Carlo method, Fiz. Tekh. Poluprovodn., 44, No. 1, 130–135 (2010).
V. G. Dubrovskii, N. V. Sibirev, and G. É. Tsyrlin, A kinetic model of the growth of nanometer filamentary crystals by the vapor–liquid–crystal mechanism, Pis’ma Zh. Tekh. Fiz., 30, Issue 16, 41–50 (2004).
M. Okuyama and J. T. Zung, Evaporation-condensation coefficient for small droplets, J. Chem. Phys. 46, 1580–1585 (1967).
V. V. Levdanskii, Dependence of the condensation (sticking) coefficient on the radius of small aerosol particles, Inzh.-Fiz. Zh., 75, No. 4, 18–22 (2002).
S. Sh. Rekhviashvili and E. V. Kishtikova, Concerning the melting temperature of nanoparticles and nanostructured substances, Pis’ma Zh. Tekh. Fiz., 32, Issue 10, 50–55 (2006).
V. V. Levdanskii, I. Smolik, and V. Zdimal, Influence of size effects on the nucleation processes in a condensed phase, Inzh.-Fiz. Zh., 84, No. 3, 531–534 (2011).
D. Kashchiev and G. M. van Rosmalen, Review: Nucleation in solutions revisited, Cryst. Res. Technol., 38, Nos. 7–8, 555–574 (2003).
W. H. Qi and M. P. Wang, Size and shape dependent melting temperature of metallic nanoparticles, Mater. Chem. Phys., 82, 280–284 (2004).
S. C. Vanithakumari and K. K. Nanda, A universal relation for the cohesive energy of nanoparticles, Phys. Lett. A, 372, 6930–6934 (2008).
W. H. Qi, M. P. Wang, M. Zhou, X. Q. Shen, and X. F. Zhang, Modeling cohesive energy and melting temperature of nanocrystals, J. Phys. Chem. Solids, 857–855 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 85, No. 5, pp. 1006–1010, September–October, 2012.
Rights and permissions
About this article
Cite this article
Levdanskii, V., Smolik, J. & Zdimal, V. Size effects during phase transformations in nanoobjects. J Eng Phys Thermophy 85, 1092–1096 (2012). https://doi.org/10.1007/s10891-012-0751-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-012-0751-5