Abstract
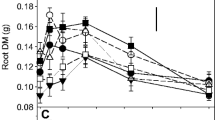
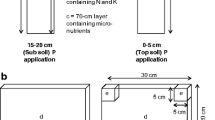
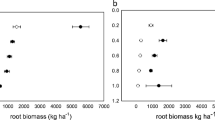
Weed-suppressive rice cultivars hold promise for improved and more economical weed management in rice. Interactions between roots of rice and weeds are thought to be modulated by the weed-suppressive activity of some rice cultivars, but these phenomena are difficult to measure and not well understood. Thus, above-ground productivity, weed suppression, and root distribution of 11 rice cultivars and two weed species were evaluated in a drill-seeded, flood-irrigated system at Stuttgart, Arkansas, USA in a two-year study. The allelopathic cultivars, PI 312777 and Taichung Native 1 (TN-1), three other weed-suppressive cultivars, three indica-derived breeding selections, and three non-suppressive commercial cultivars were evaluated in field plots infested with barnyardgrass (Echinochloa crus-galli (L.) Beauv.) or bearded sprangletop (sprangletop, Leptochloa fusca (L.) Kunth var. fascicularis (Lam.) N. Snow). The allelopathic cultivars produced more tillers and suppressed both weed species to a greater extent than did the breeding selections or the non-suppressive cultivars. 13C isotope discrimination analysis of mixed root samples to a depth of 15 cm revealed that the allelopathic cultivars typically produced a greater fraction of their total root mass in the surface 0–5 cm of soil depth compared to the breeding selections or the non-suppressive cultivars, which tended to distribute their roots more evenly throughout the soil profile. These trends in root mass distribution were apparent at both early (pre-flood) and late-season stages in weed-free and weed-infested plots. Cultivar productivity and root distribution generally responded similarly to competition with the two weed species, but barnyardgrass reduced rice yield and root mass more than did sprangletop. These findings demonstrate for the first time that roots of the allelopathic cultivars PI 312777 and TN-1 explore the upper soil profile more thoroughly than do non-suppressive cultivars under weed-infested and weed-free conditions in flood-irrigated U.S. rice production systems. They raise the interesting prospect that root proliferation near the soil surface might enhance the weed-suppressive activity of allelochemical exudates released from roots. Plant architectural design for weed suppressive activity should take these traits into consideration along with other proven agronomic traits such as high tillering and yield.





Similar content being viewed by others
References
Akhter, J., Mahmood, K., Tasneem, M. A., Naqvi, M. H., and Malik, K. A. 2003. Comparative water-use efficiency of Sporobolus arabicus and Leptochloa fusca and its relation with carbon isotope discrimination under semiarid conditions. Plant Soil 249:263–269.
Chauhan, B. S. and Johnson, D. E. 2010a. Relative importance of shoot and root competition in dry-seeded rice growing with junglerice (Echinochloa colona) and ludwigia (Ludwigia hyssopifolia). Weed Sci. 58:295–299.
Chauhan, B. S. and Johnson, D. E. 2010b. Responses of rice flatsedge (Cyperus iria) and barnyardgrass (Echinochloa crus-galli) to rice interference. Weed Sci. 58:204–208.
Chauhan, B. S. and Johnson, D. E. 2011. Phenotypic plasticity of Chinese sprangletop (Leptochloa chinensis) in competition with seeded rice. Weed Technol. 25:652–658.
Chen, X. H., Hu, F., and Kong, C. H. 2008. Varietal improvement in rice allelopathy. Allelopathy J. 22:379–384.
Chung, I. M., Kim, J. T., and Kim, S. H. 2006. Evaluation of allelopathic potential and quantification of momilactone A,B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J Agric Food Chem 54:2527–2536.
Clark, R., Maccurdy, R., Jung, J., Shaff, J., McCouch, S., Aneshansley, D., and Kochian, L. 2011. Three-dimensional root phenotyping with a novel imaging and software platform. Plant Physiol 156:455–465.
Derner, J. D., Johnson, H. B., Kimball, B. A., Pinter, P. J. J. R., Polley, H. W., Tischler, C. R., Boutton, T. W., Lamorte, R. L., Wall, G. W., Adam, N. R., Leavitt, S. W., Ottman, M. J., Matthias, A. D., and Brooks, T. J. 2003. Above- and below-ground responses of C3–C4 species mixtures to elevated CO2 and soil water availability. Glob. Change Biol. 9:452–460.
Dilday, R. H., Mattice, J. D., Moldenhauer, K. A., and Yan, W. 2001a. Allelopathic potential in rice germplasm against ducksalad, redstem and barnyardgrass. J Crop Prod 4:287–301.
Dilday, R. H., Yan, W. G., Moldenhauer, K. A., Gibbons, J. W., Lee, F. N., and Bryant, R. J. 2001b. Chinese and other foreign germplasm evaluation, pp. 1–12, in R. J. Norman and J.-F. Meullenet (eds.), Bobby R. Wells rice research studies 2000. Arkansas Agricultural Experiment Station, Series 485. University of Arkansas, Fayetteville.
Dingkuhn, M., Farquhar, G. D., De Datta, S. K., and O’toole, J. C. 1991. Discrimination of 13C among upland rices having different water use efficiencies. Aust. J. Agric. Res. 42:1123–1131.
Dingkuhn, M., Johnson, D. E., Sow, A., and Audebert, A. Y. 1999. Relationships between upland rice canopy characteristics and weed competitiveness. Field Crop Res. 61:79–95.
Ehleringer, J. R. 1991. 13C/12C fractionation and its utility in terrestrial plant studies, pp. 187–200, in D. C. Coleman and B. B. Fry (eds.), Carbon isotope techniques. Academic Press, San Diego.
Eleki, K., Cruse, R. M., and Albrecht, K. A. 2005. Root segregation of C3 and C4 species using carbon isotope composition. Crop Sci 45:879–882.
Farquhar, G. D., Ehleringer, J. R., and Hubick, K. T. 1989. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40:503–537.
Fischer, A., Ramirez, H. V., and Lozano, J. 1997. Suppression of junglerice [Echinochloa colona (L.) Link] by irrigated rice cultivars in Latin America. Agron J 89:516–521.
Fofana, B. and Rauber, R. 2000. Weed suppression ability of upland rice under low-input conditions in West Africa. Weed Res. 40:271–280.
Gealy, D. R. and Fischer, A. J. 2010. 13C discrimination: a stable isotope method to quantify root interactions between C3 rice (Oryza sativa) and C4 barnyardgrass (Echinochloa crus-galli) in flooded fields. Weed Sci. 58:359–368.
Gealy, D. R. and Gealy, G. S. 2011. 13Carbon isotope discrimination in roots and shoots of major weed species of southern U.S. rice fields and its potential use for analysis of rice–weed root interactions. Weed Sci 59:587–600.
Gealy, D. R. and Moldenhauer, K. A. 2005. Progress in developing weed suppressive rice cultivars for the southern U.S, pp. 257–296, in H. Singh, D. Batish, and R. Kohli (eds.), Handbook of sustainable weed management. Haworth Press, Binghampton.
Gealy, D. R. and Moldenhauer, K. A. K. 2012. Use of 13C isotope discrimination analysis to quantify distribution of barnyardgrass and rice roots in a four-year study of weed-suppressive rice. Weed Sci. 60:133–142.
Gealy, D. R. and Yan, W. G. 2012. Weed suppression potential of ‘Rondo’ and other indica rice germplasm lines. Weed Technol. 26:(in press doi:10.1614/WT-D-11-00141.1)
Gealy, D. R., Moldenhauer, K. A. K, and Jia, M. H. 2013. Field performance of STG06L-35-061, a new genetic resource developed from crosses between weed-suppressive indica rice and commercial southern U.S. long-grains. Plant Soil: in press.
Gealy, D. R., Wailes, E. J., Estorninos, L. E. Jr., and Chavez, R. S. C. 2003. Rice cultivar differences in suppression of barnyardgrass (Echinochloa crus-galli) and economics of reduced propanil rates. Weed Sci. 51:601–609.
Gealy, D., Ottis, B., Talbert, R., Moldenhauer, K., and Yan, W. 2005. Evaluation and improvement of allelopathic rice germplasm at Stuttgart, Arkansas, USA, pp. 157–163 in: Proceedings of the 4th World Congress on Allelopathy. Wagga Wagga, NSW, Australia: International Allelopathy Society.
Gibson, K. D., Foin, T. C., and Hill, J. E. 1999. The relative importance of root and shoot competition between water-seeded rice and Echinochloa phyllopogon. Weed Res. 39:181–190.
Gibson, K. D., Hill, J. E., Foin, T. C., Caton, B. P., and Fischer, A. J. 2001. Water-seeded rice cultivars differ in ability to interfere with watergrass. Agron J 93:181–190.
Gibson, K. D., Fischer, A. J., Foin, T. C., and Hill, J. E. 2003. Crop traits related to weed suppression in water-seeded rice (Oryza sativa L.). Weed Sci 51:87–93.
Gravois, K. A., Moldenhauer, K. A. K., Lee, F. N., Norman, R. J., Helms, R. S., Bernhardt, J. L., Wells, B. R., Dilday, R. H., Rohman, P. C., and Blocker, M. M. 1995. Registration of 'Kaybonnet' rice. Crop Sci 35:587–588.
Iyer-Pascuzzi, A., Symonova, O., Mileyko, Y., Hao, Y., Belcher, H., Harer, J., Weitz, J., and Benfey, P. 2010. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiol 152:1148–1157.
Kato-Noguchi, H. 2011. Barnyardgrass-induced rice allelopathy and momilactone B. J. Plant Physiol. 168:1016–1020.
Kato-Noguchi, H. and Peters, R. (2013). The role of momilactones in rice allelopathy, J. Chem. Ecology. 39:175–185.
Kato-Noguchi, H., Kujimea, H., and Inoa, T. 2007. UV-induced momilactone B accumulation in rice rhizosphere. J. Plant Physiol. 164:1548–1551.
Kim, S. Y., Madrid, A. V., Park, S. T., Yang, S. J., and Olofsdotter, M. 2005. Evaluation of rice allelopathy in hydroponics. Weed Res. 45:74–79.
Kodama, O., Suzuki, T., Miyakawa, J., and Akatsuka, T. 1988. Ultraviolet-induced accumulation of phytoalexins in rice leaves. Agric Biol Chem 52:2469–2473.
Kondo, M., Pablico, P. P., Aragones, D. V., and Agbisit, R. 2004. Genotypic variations in carbon isotope discrimination, transpiration efficiency, and biomass production in rice as affected by soil water conditions and N. Plant Soil 267:165–177.
Kong, C., Liang, W., Xu, X., Hu, F., Wang, P., and Jiang, Y. 2004a. Release and activity of allelochemicals from allelopathic rice seedlings. J Agric Food Chem 52:2861–2865.
Kong, C., Xu, X., Zhou, B., Hua, F., Zhang, C., and Zhang, M. 2004b. Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens. Phytochemistry 65:1123–1128.
Kong, C. H., Li, H. B., Hu, F., Xu, X. H., and Wang, P. 2006. Allelochemicals released by rice roots and residues in soil. Plant Soil 288:47–56.
Kong, C. H., Hu, F., Wang, P., and Wu, J. L. 2008. Effect of allelopathic rice varieties combined with cultural management options on paddy field weeds. Pest Manag. Sci. 64:276–282.
Kong, C. H., Chen, X. H., Hu, F., and Zhang, S. Z. 2011. Breeding of commercially acceptable allelopathic rice cultivars in China. Pest Manag. Sci. 67:1100–1106.
Laza, M. R., Kondo, M., Ideta, O., Barlaan, E., and Imbe, T. 2006. Identification of quantitative trait loci for δ13C and productivity in irrigated lowland rice. Crop Sci 46:763–773.
Ma, H. J., Shin, D. H., Lee, I. J., Koh, J. C., Park, S. K., and Kim, K. U. 2006. Allelopathic K21 selected as promising allelopathic rice. Weed Biol. Manag. 6:189–196.
McClung, A. M., Fjellstrom, R. G., Bergman, C. J., Bormans, C. A., Park, W. D., and Marchetti, M. A. 2004. Registration of ‘Saber’ rice. Crop Sci 44:693–694.
Moldenhauer, K. A. K., Lee, F. N., Bernhardt, J. L., Norman, R. J., Staton, N. A., Wilson, C. E., Anders, M. M., Cartwright, R. D., and Blocker, M. M. 2007. Registration of ‘Wells’ Rice. Crop Sci 47:442–443.
O’barr, J. H., McCauley, G. N., Bovey, R. W., Senseman, S. A., and Chandler, J. M. 2007. Rice response to clomazone as influenced by application rate, soil type, and planting date. Weed Technol. 21:199–205.
Peng, S., Laza, R. C., Khush, G. S., Sanico, A. L., Visperas, R. M., and Garcia, F. V. 1998. Transpiration efficiencies of indica and improved tropical japonica rice grown under irrigated conditions. Euphytica 103:103–108.
Perera, K. K., Ayers, P. G., and Gunasena, H. P. M. 1992. Root growth and the relative importance of root and shoot competition in interactions between rice (Oryza sativa) and Echinochloa crus-galli. Weed Res. 32:67–76.
Pérez de Vida, F. B., Laca, e., Mackill, D., Fernandez, G. M., and Fischer, A. 2006. Relating rice traits to weed competitiveness and yield: a path analysis. Weed Sci. 54:1122–1131.
Polley, H. W., Johnson, H. B., and Mayeux, H. S. 1992. Determination of root biomasses of three species grown in a mixture using stable isotopes of carbon and nitrogen. Plant Soil 142:97–106.
Rewald, B., Meinen, C., Trockenbrodt, M., Ephrath, J. E., and Rachmilevitch, S. 2012. Root taxa identification in plant mixtures- current techniques and future challenges. Plant Soil. doi:10.1007/s11104-012-1164-0.
Scartazza, A., Lauteri, M., Guido, M. C., and Brugnoli, E. 1998. Carbon isotope discrimination in leaf and stem sugars, water-use efficiency and mesophyll conductance during different developmental stages in rice subjected to drought. Aust J Plant Physiol 25:489–498.
Scott, R. C., Boyd, J. W., Smith, K. L., Selden, G., and Norsworthy, J. K. 2012. Recommended chemicals for weed and brush control. MP-44. University Arkansas Extension and U.S. Department of Agriculture. p. 78–91.
Seal, A. N. and Pratley, J. E. 2010. The specificity of allelopathy in rice (Oryza sativa). Weed Res. 50:303–311.
Shin, D. H., Kim, K. U., Sohn, D. S., Kang, S. U., Kim, H. Y., Lee, I. J., and Kim, M. Y. 2000. Regulation of gene expression related to allelopathy, pp. 109–124, in K. U. Kim and D. H. Shin (eds.), Rice allelopathy. Kyungpook National University, Taegu, Korea.
Smith Jr., R. J. 1988. Weed thresholds in southern U.S. rice (Oryza sativa). Weed Technol 2:232–241.
Svejcar, T. J. and Boutton, T. W. 1985. The use of stable carbon isotope analysis in rooting studies. Oecologia 67:205–208.
Svejcar, T. J., Boutton, T. W., and Christiansen, S. 1988. Rooting dynamics of Medicago sativa seedlings growing in association with Bothriochloa caucasica. Oecologia 77:453–456.
Wang, Y. P., Tang, L. H., Zhang, H. S., and Fang, X. W. 2005. Induction effect of some weeds on the allelopathy of rice varieties. Chin. Ecol. Environ. 14:250–252.
Webster, T. M. 2000. The southern states 10 most common and troublesome weeds in rice. Proc. South. Weed Sci. Soc. 53:247–274.
Wilson, J. B. 1988. Shoot competition and root competition. J Appl Ecol 25:279–296.
Worthington, M. and Reberg-Horton, S. C. 2013. Breeding cereal crops for enhanced weed suppression: optimizing allelopathy and competitive ability. J. Chem. Ecol. 39:213–231.
Xu, Y., This, D., Pausch, R. C., Vonhof, W. M., Coburn, J. R., Comstock, J. P., and McCouch, S. R. 2009. Leaf-level water use efficiency determined by carbon isotope discrimination in rice seedlings: genetic variation associated with population structure and QTL mapping. Theor Appl Genet 118:1065–1081.
Yan, W. G. and McClung, A. M. 2010. ‘Rondo’, a long-grain indica rice with resistances to multiple diseases. J. Plant Reg. 4:131–136.
Zhao, D. L., Atlin, G. N., Bastiaans, L., and Spiertz, J. H. J. 2006. Developing selection protocols for weed competitiveness in aerobic rice. Field Crop Res. 97:272–285.
Acknowledgments
Thanks to Howard Black for technical assistance and data and statistical analyses; Adam Davis, Gordon Miller, Jim Gignac, and Kenneth Hale for plant sampling, cleaning, and grinding; Erik Pollock with the University of Arkansas Stable Isotope Laboratory, Fayetteville, AR, <http://biology.uark.edu/uasil/home.html> for conducting 13C isotope discrimination analyses; and Doug Boyette, David Clay, and Albert Fischer for helpful reviews.
The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual's income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 24.4 kb)
Rights and permissions
About this article
Cite this article
Gealy, D., Moldenhauer, K. & Duke, S. Root Distribution and Potential Interactions Between Allelopathic Rice, Sprangletop (Leptochloa spp.), and Barnyardgrass (Echinochloa crus-galli) based on 13C Isotope Discrimination Analysis. J Chem Ecol 39, 186–203 (2013). https://doi.org/10.1007/s10886-013-0246-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0246-7